Marburg Vaccine Candidate Deployed in Ethiopia

In an effort to reduce the ongoing Marburg Virus Disease (MVD) outbreak in Ethiopia, the Sabin Vaccine Institute has announced that it has sent about 640 doses of its investigational cAd3-Marburg Vaccine to Ethiopia.
As of December 5, 2025, there are currently no licensed vaccines or treatments for MVD.
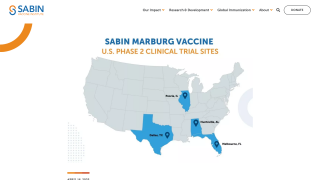
Sabin's single-dose cAd3-Marburg Vaccine is being used in a Phase 2 outbreak clinical trial in Rwanda; the vaccine is also in Phase 2 clinical trials in the U.S., Uganda, and Kenya.
"The Ethiopian Ministry of Health reached out to Sabin early in the outbreak, and we have been working in partnership to support Minister of Health Dr. Mekdes Daba and her team," says Sabin Chief Executive Officer Amy Finan, in a press release.
"We've built on our previous outbreak response experience to assist our Ethiopian colleagues as requested quickly."
The Biomedical Advanced Research and Development Authority, part of the U.S. Department of Health and Human Services' Administration for Strategic Preparedness and Response, funds the development and manufacture of Sabin's investigational vaccine candidate.
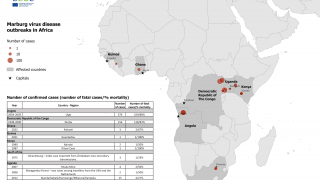
Ethiopia's Ministry of Health recently confirmed 13 Marburg infections, including eight deaths, in its latest update.
Our Trust Standards: Medical Advisory Committee