Lyme Disease Vaccination Benefits Confirmed
Valneva SE today announced positive final results regarding the immunogenicity and safety from its Phase 2 study, VLA15-221, of the Lyme disease vaccine candidate, VLA15.
According to Valneva's press release on November 26, 2025, this study's findings demonstrated a strong anamnestic immune response and a favorable safety profile six months after the third booster dose (at month 48) across all age groups.
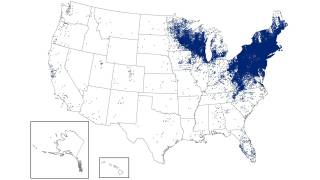
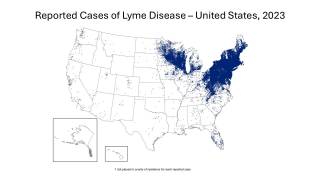
Furthermore, these results confirm that the vaccine is compatible with the expected benefits of annual vaccination before the start of each Lyme season, which coincides with increased outdoor activities in the Northeast and Midwest regions of the United States.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release. "Lyme disease continues to expand geographically and remains a pressing unmet medical need affecting communities across the Northern Hemisphere."
"Each set of positive results moves us closer to the possibility of making this vaccine available to adults, adolescents, and children living in Lyme-endemic areas."
This is very encouraging news as there are currently no approved human vaccines for Lyme disease, and VLA15 has advanced the furthest in clinical development.
Valneva partnered with Pfizer Inc. in April 2020 to develop and commercialize VLA15.
Our Trust Standards: Medical Advisory Committee