US FDA To Authorize Emergency Use of Animal Drugs to Reduce New World Screwworm Threat
The U.S. Department of Health and Human Services recently issued a declaration that allows the U.S. Food and Drug Administration (FDA) to issue Emergency Use Authorizations for animal drugs to treat or prevent infestations caused by the New World Screwworm (NWS).
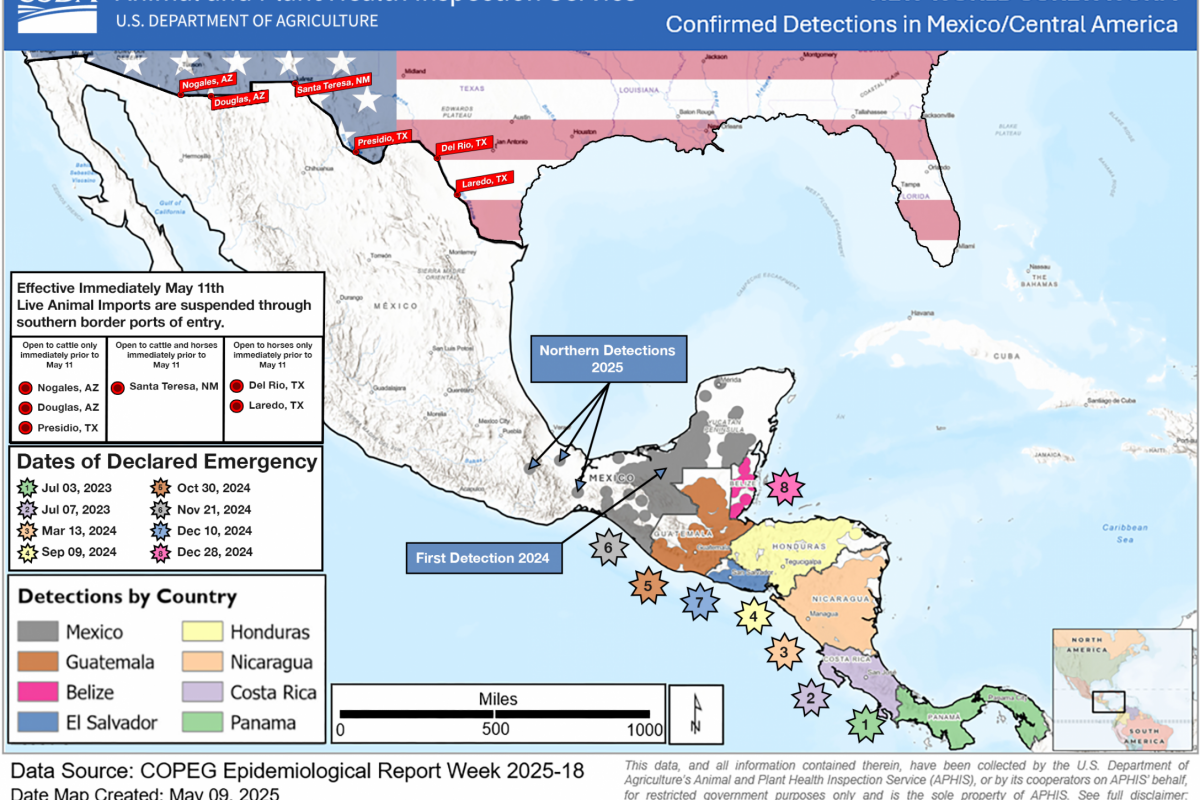
As of August 20, 2025, there are no FDA-approved drugs for NWS, nor are there vaccines authorized to protect people in the United States.
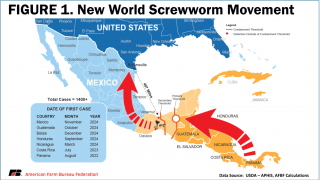
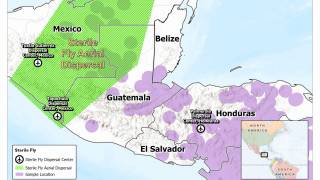
As of today, NWS infestations have been confirmed in Central America and Mexico, but not in Texas.
The FDA stated in a press release that the parasite's risk to human health in the United States remains very low. Still, the potential future threat to animal populations and the food supply chain requires proactive action.
“Our priority is to safeguard both animal health and the nation’s food supply,” said FDA Commissioner Marty Makary, M.D., M.P.H., in a press release.
“FDA is acting swiftly and responsibly to help ensure we have the necessary tools to prevent and control New World Screwworm, minimizing risks to agriculture and public health.”
Over the last two months, about $900 million has been committed by the U.S. government to combat the NWS from reaching the U.S.
NWS infests warm-blooded animals, including livestock, pets, wildlife, and, in rare cases, humans, causing severe tissue damage and sometimes death, writes the FDA.
Our Trust Standards: Medical Advisory Committee