Chikungunya Vaccine Candidate Seeks Europe's Accelerated Assessment
Valneva SE today announces the submission of a marketing application with the European Medicines Agency (EMA) for approval of the Company's single-shot chikungunya vaccine candidate, VLA1553.
According to Valneva's press release on October 25, 2023, VLA1553 was also granted accelerated assessment for the application by EMA's Committee for Medicinal Products for Human Use based on the vaccine candidate's "major interest for public health and therapeutic innovation."
If approved in Europe, VLA1553 could become the first licensed chikungunya virus (CHIKV) vaccine to address this unmet medical need.
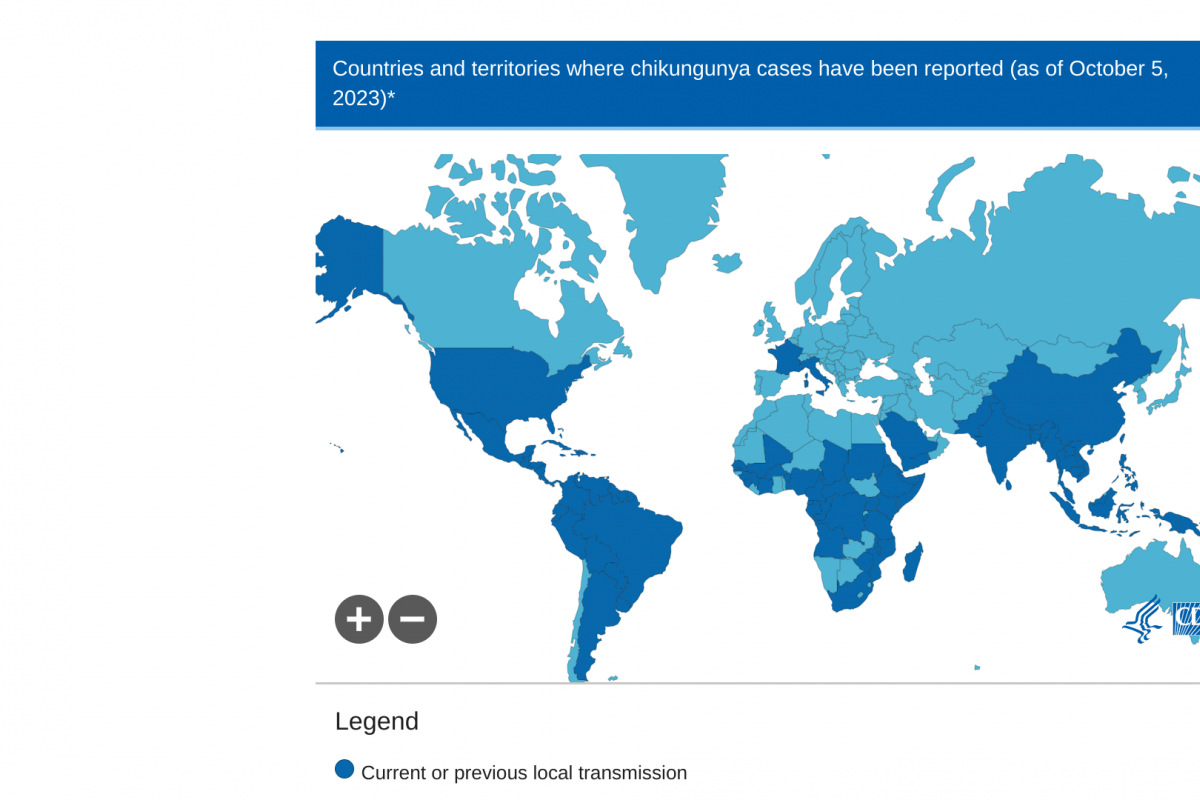
As of August 2023, approximately 320,000 CHIKV cases and over 340 related deaths have been reported worldwide by the European Centre for Disease Prevention and Control (ECDC).
VLA1553 is currently the first and only chikungunya vaccine candidate worldwide for which regulatory review processes are underway.
A Biologic License Application (BLA) is currently under priority review by the U.S. Food and Drug Administration, with a Prescription Drug User Fee Act action date planned for the end of 2023.
Additionally, a marketing application is under review by Health Canada.
Juan Carlos Jaramillo, MD, Chief Medical Officer of Valneva, commented, "Chikungunya virus is a serious and debilitating mosquito-borne viral infection that poses a significant unmet need, and the risk of chikungunya spreading in Europe is relatively high due to the possibility of infected travelers."
"No vaccine or specific treatments are currently available for this debilitating disease. We will continue to work diligently to bring VLA1553 to different territories as soon as possible."
Additionally, other CHIKV vaccine candidates are conducting clinical trials in 2023.
During 2023, chikungunya outbreaks were primarily found in Africa, the Americas, Asia, Brazil, and the Indian subcontinent. In January 2023, the U.S. Centers for Disease Control and Prevention reported 81 travel-associated CHIKV cases in the U.S. in 2022.
Our Trust Standards: Medical Advisory Committee























