Dengue Tetravalent Vaccine Approved Regardless of Prior Dengue Exposure

Japan-based Takeda today announced the company's dengue vaccine, QDENGA®, was approved by the Indonesia National Agency for Drug and Food Control, Badan Pengawas Obat dan Makanan, for the prevention of dengue disease caused by any serotype in individuals six years to 45 years of age.
QDENGA (Dengue Tetravalent Vaccine [Live, Attenuated]) (TAK-003) is the only dengue vaccine approved in Indonesia for use in individuals six years to 45 years of age regardless of previous dengue exposure and without the need for pre-vaccination testing.
In Indonesia, QDENGA should be administered subcutaneously as a 0.5 mL dose at a two-dose (0 and 3 months) schedule.
"Dengue can affect anyone living in or traveling to endemic areas - regardless of age, health, and socio-economic circumstances," said Gary Dubin, president of Takeda's Vaccine Business Unit, in a press release on August 22, 2022.
"Developing this innovative dengue vaccine has been an exciting challenge, and its approval in Indonesia is an important achievement for Takeda and for public health."
"We're proud to introduce QDENGA as a new dengue prevention tool to the people of Indonesia, and we will continue to work with additional regulatory agencies to make QDENGA."
QDENGA was assessed across a robust clinical development program that included various Phase 1, Phase 2, and Phase 3 trials and more than 28,000 participants, including Takeda's pivotal Tetravalent Immunization against Dengue Efficacy Study (TIDES) trial.
The TIDES trial met its primary endpoint of overall vaccine efficacy against virologically-confirmed dengue with 80.2% efficacy at a 12-month follow-up.
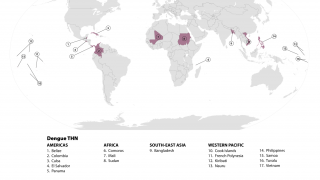
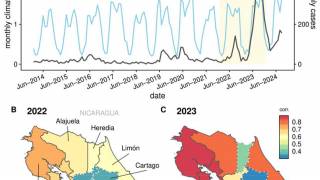
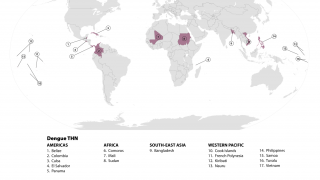
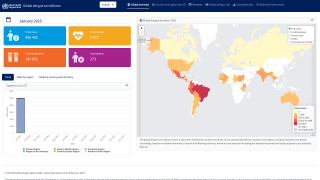
Dengue is a mosquito-borne viral disease that poses a significant global public health threat, with prevalence in over 125 countries.
Recovery from infection by one dengue serotype provides lifelong immunity against only that serotype.
However, later exposure to any of the remaining serotypes might be associated with an increased risk of severe disease.
In the first half of 2022 alone, Indonesia reported over 63,000 dengue cases and nearly 600 related fatalities.
As of August 15, 2022, the U.S. CDC stated 'that dengue is a risk in many parts of Central and South America, Mexico, and the Caribbean. These countries are reporting increased numbers of cases of the disease.
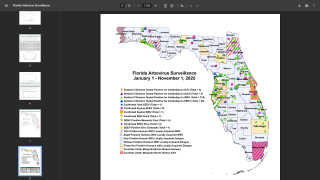
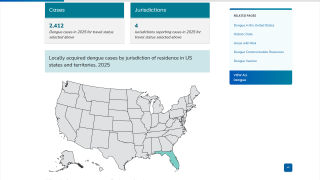
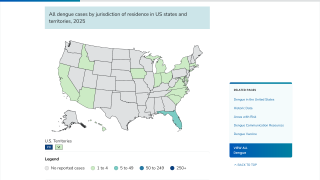
And in the USA, the Florida Department of Health in Miami-Dade County recently confirmed that it remains under a mosquito-borne illness advisory following the identification of the third local dengue infection in 2022.
With today's approval, there are now two dengue vaccines available.
The U.S. FDA authorized the Dengvaxia vaccine in 2019.
Additional dengue vaccine news is posted at Vax-Before-Travel.com/Dengue.
The company's press release was manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee