Florida health authorities have confirmed the first locally acquired case of chikungunya in 2026, occurring in Miami-Dade County.
This incident underscores the ongoing risks posed by this mosquito-borne virus, particularly given the significant number of travel-related cases.
For example, just a few days ago, thousands of travelers visited Miami-Dade to attend the NCAA football championship game.
As of January 17, 2026, the Florida Department of Health (DOH) reported 16 cases of chikungunya associated with travel. In the previous year, the state recorded 328 cases, with Miami-Dade County accounting for 214, significantly surpassing all other areas.
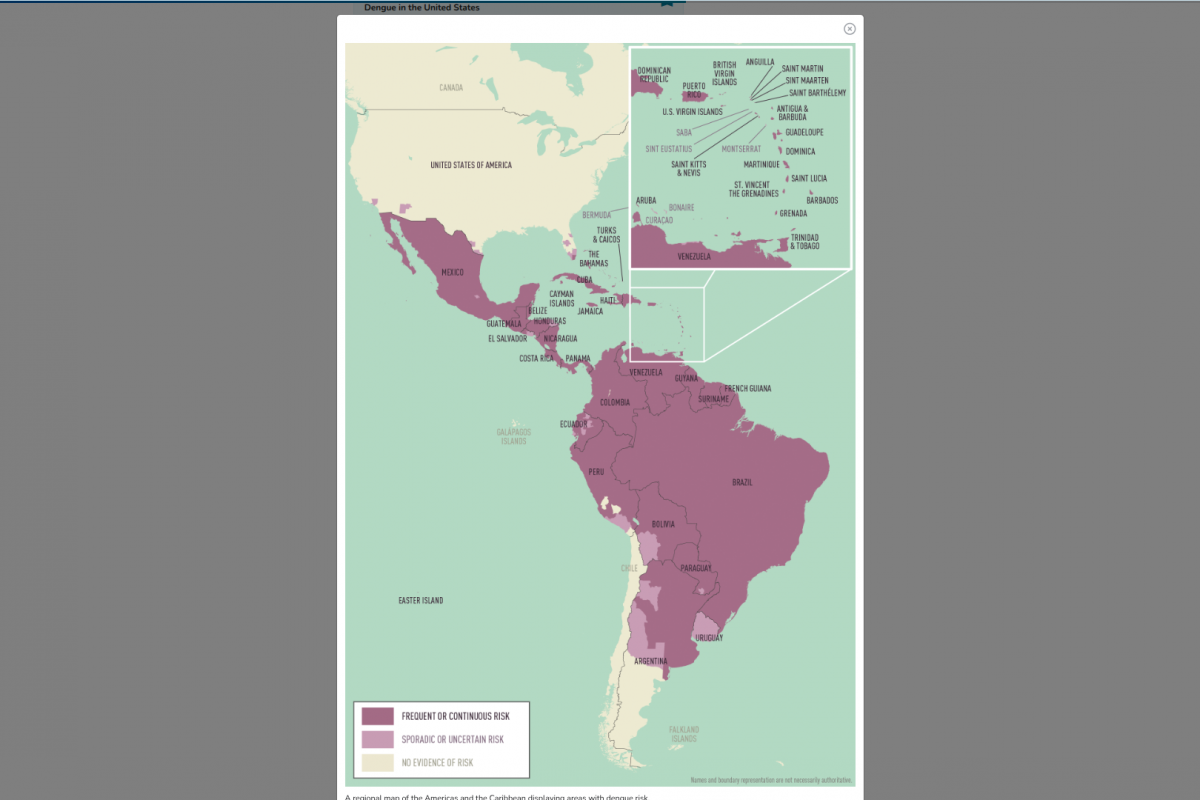
The vast majority (316) of Florida's travel-related cases originated from travelers returning from Cuba, where the virus has surged dramatically.
Cuba has reported more than 50,000 chikungunya cases in 2025, with all 15 provinces affected.
Nationally, the U.S. Centers for Disease Control and Prevention (CDC) has seen more than 400 chikungunya cases in 2025, with all but the two locally acquired instances linked to travel.
The CDC says the chikungunya virus, transmitted primarily by Aedes mosquitoes, causes symptoms in most infected individuals within 3–7 days of a bite. Common signs include high fever and severe joint pain, often debilitating. While the majority recover within a week, some experience persistent or chronic joint pain lasting months or even years.
As of January 21, 2026, there is no specific antiviral treatment for chikungunya.
However, a chikungunya vaccine has been approved by the U.S. FDA and is commercially available at travel clinics across the United States and in various other countries.
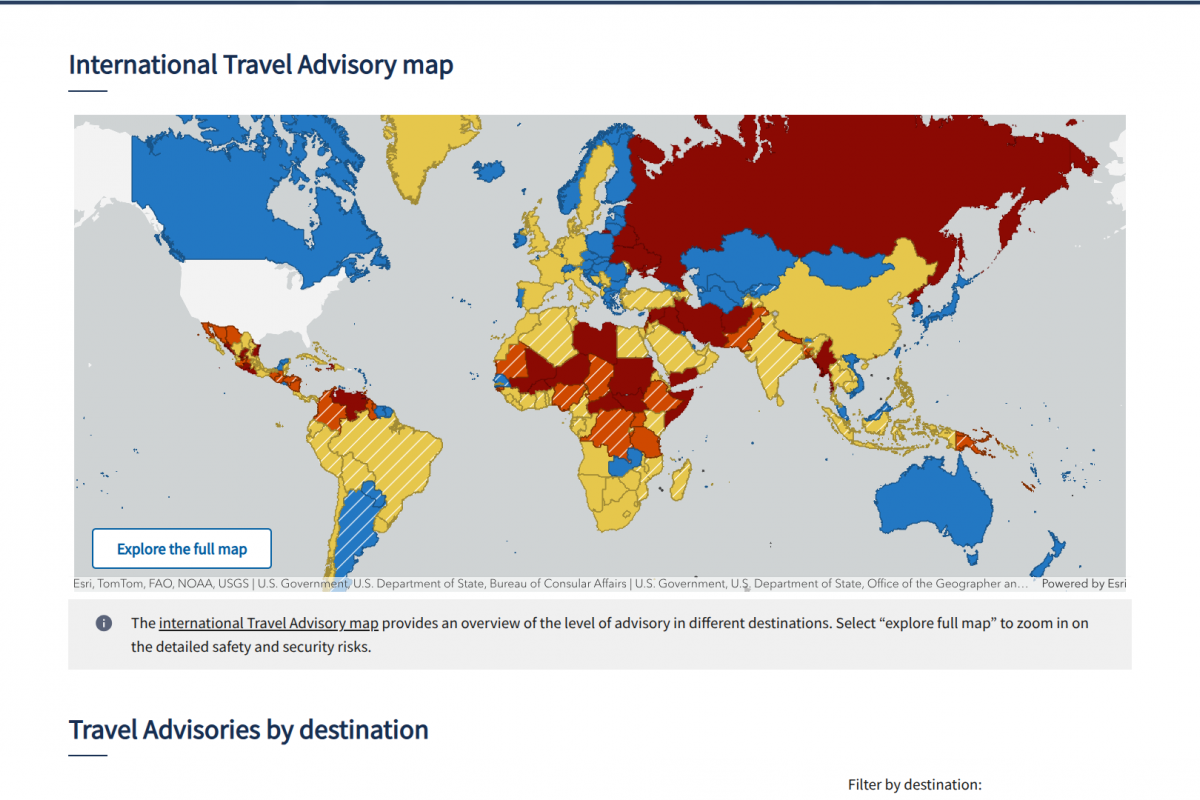
The CDC urges travelers to consult healthcare providers or travel medicine specialists before trips to affected destinations, use insect repellents, wear protective clothing, and eliminate standing water to reduce mosquito breeding.
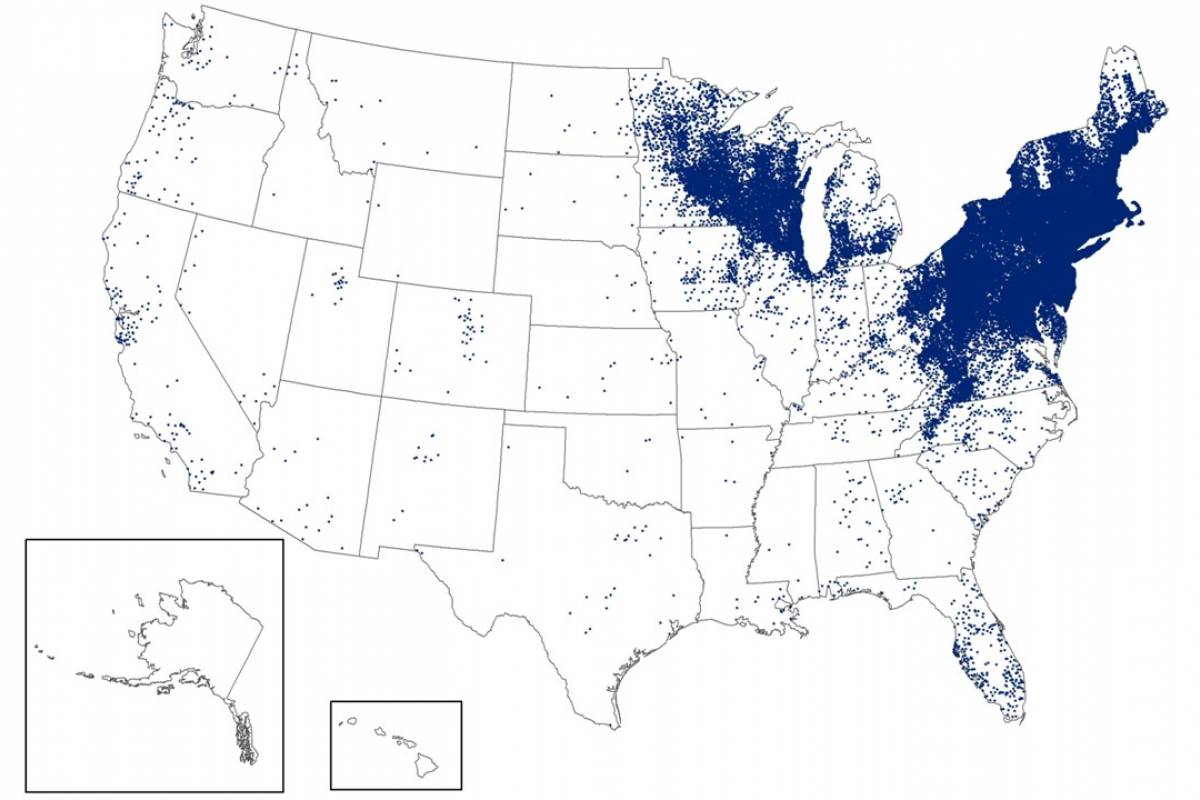
Visitors to and residents in areas like South Florida should also maintain vigilance against local vectors, especially given the presence of competent Aedes mosquitoes.