The Republic of Indonesia and the World Health Organization (WHO) recently declared the end of its poliovirus type 2 outbreak, which was caused by years of low polio immunization coverage.
The outbreak began in October 2022, when the first confirmed case was reported in Aceh province. Over the next two years, polio cases appeared in the provinces of Banten, West Java, Central Java, East Java, North Maluku, Central Papua, Highland Papua, and South Papua.
The last confirmed case was in South Papua on June 27, 2024.
During the response, nearly 60 million additional doses of the polio vaccine were administered to children.
Indonesia's response included two rounds of nationwide polio campaigns using novel OPV-2 (nOPV2) vaccine between the end of 2022 and the third trimester of 2024.
In parallel, routine immunization coverage also improved, with the percentage of children receiving their second dose of inactivated polio vaccine (IPV) rising from 63% (1.9 million children) in 2023 to 73% (3.2 million children) in 2024.
"Indonesia's success marks a vital step towards a polio-free world. It also reinforces the entire WHO Western Pacific Region's ability to retain its polio-free status, an achievement we proudly reached 25 years ago," noted Dr Saia Ma'u Piukala, WHO Regional Director for the Western Pacific, in a press release on November 21, 2025.
"I urge all our 38 countries and areas to remain vigilant."
"One day, polio will be just a memory. Until then, we vaccinate."
As of late 2025, over 2 billion doses of the nOPV2 vaccine have been administered globally.
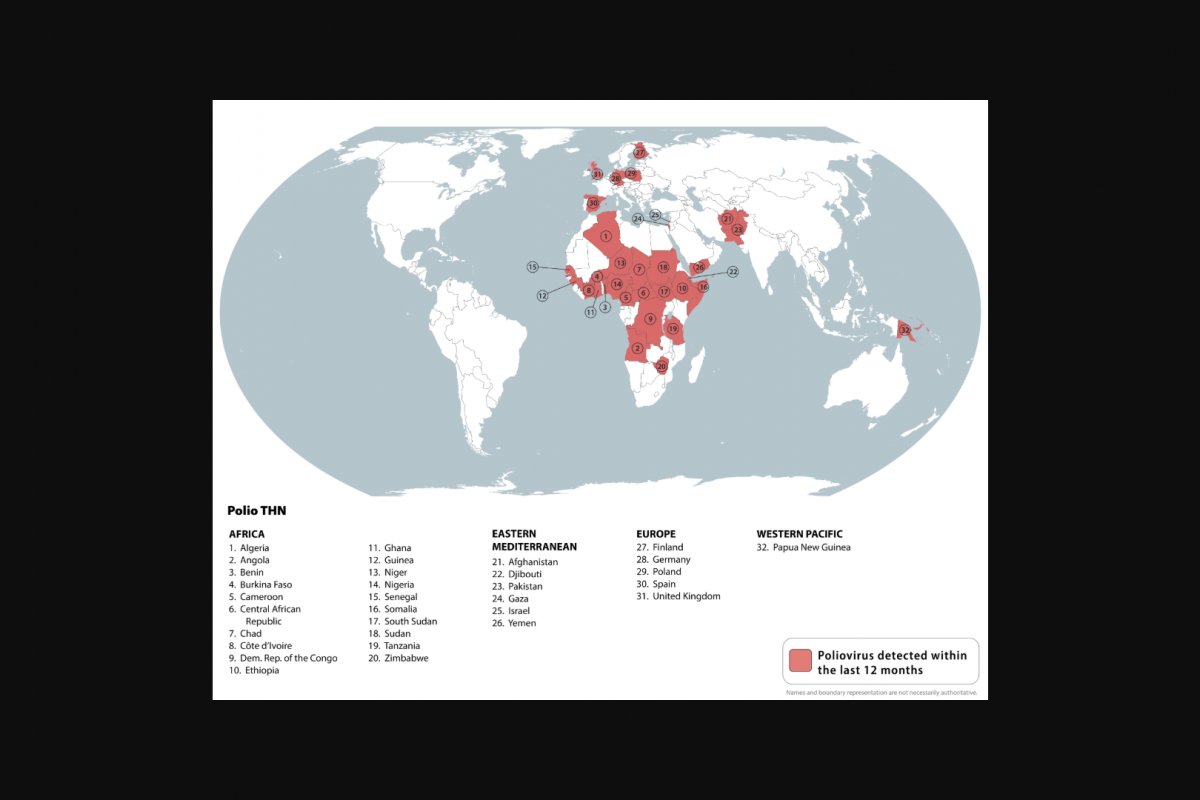
To highlight this risk to international travelers, the U.S. CDC updated its Level 2 - Practice Enhanced Precautions notice, which identifies 32 countries, and suggested IPV booster doses for certain people.