Severe Chikungunya Manifestations Confirmed in 20% of Hospitalized Children

Over the past decade, the World Health Organization has confirmed that Chikungunya virus (CHIKV) outbreaks have occurred frequently in about 110 countries. In 2024, outbreaks significantly exceeded previous years, severely impacting both adults and children.
One reason for this uptick in cases may be a CHIKV mutation.
Since 2019, Southeast Asian countries have detected the Chikungunya virus East/Central/South African-derived genotype with E1:A226V and E1:K211E mutations. This mutated strain has exhibited enhanced infectivity, dissemination, and transmission.
Research has suggested that this genotype triggers a higher inflammatory response and pathology than the Asian-derived genotype responsible for the 2014–2015 Caribbean CHIKV outbreak.
A new study published by the Pediatric Infectious Diseases Journal in February 2025 aimed to describe clinical manifestations and complications of CHIKV in children during Thailand's recent outbreak.
This study reported that 20% of hospitalized children had severe chikungunya manifestations.
In this new study, CHIKV-associated arthralgia during the Asian-derived genotype indicated a prevalence of arthralgia of 88% in children and 98% in adults, with prevalence increasing age-dependent.
These researchers wrote, 'This discrepancy can be attributed to differences in genotypes or to the nature of our retrospective review, which could have led to underreporting of symptoms during hospital visits.'
Although most of the infected children had mild to moderate symptoms and typically resolved within 5–7 days that required only supportive care, the manifestations that raised concerns were shock, seizures, and altered mental status that required intensive care, which was found in 20% of hospitalized children in our cohort.
In this study, 4% of cases developed shock.
A previous case series in adults indicates that CHIKV-associated severe sepsis or shock in adults has a mortality rate of up to 48%.
Undetected shock in children with severe CHIKV can lead to multiorgan failure.
Moreover, neurological manifestations were found in 8% of patients.
These researchers concluded that clinical evaluation and laboratory findings are crucial for CHIKV case investigation and management.
Alongside avoiding infected mosquito bites, vaccination has become a viable option against this mosquito-transmitted disease.
Over the past two years, the first CHIKV vaccine has received approval from the U.S. Food and Drug Administration and European Medicines Agency for adults at risk of exposure to CHIKV, including international travelers planning to visit endemic areas.
Furthermore, regulatory approval for children may be forthcoming in the coming months.
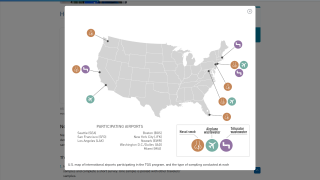
In 2025, most travel vaccine clinics and pharmacies in the United States commercially offer Valneva SE's IXCHIQ® chikungunya vaccine.
Our Trust Standards: Medical Advisory Committee