Recently, the World Health Organization and the Africa Centres for Disease Control and Prevention identified Rift Valley fever as a priority disease.
To address this need, a promising vaccine candidate against the potentially deadly Rift Valley fever is set to begin Phase II trials in Kenya. ChAdOx1 RVF is the most advanced stage of testing a human Rift Valley fever vaccine candidate.
While Rift Valley fever vaccines have been registered for animals, no vaccines are available for human use.
ChAdOx1 RVF has already shown positive results in the first stage of clinical trials conducted in the UK. The trial demonstrated that the vaccine was safe and well-tolerated in volunteers who received a single shot of the vaccine, and that it elicited high levels of neutralising antibodies which block viral infection and mediate protection against the virus.
Studies have also shown that the vaccine protects against Rift Valley fever in multiple livestock species, suggesting that it could be used for both people and livestock.
“Rift Valley fever disproportionately affects the lives and livelihoods of vulnerable pastoral communities, potentially causing both human fatalities and large-scale livestock losses,” said Dr. Richard Hatchett, CEO of the Coalition for Epidemic Preparedness Innovations (CEPI), in a press release.
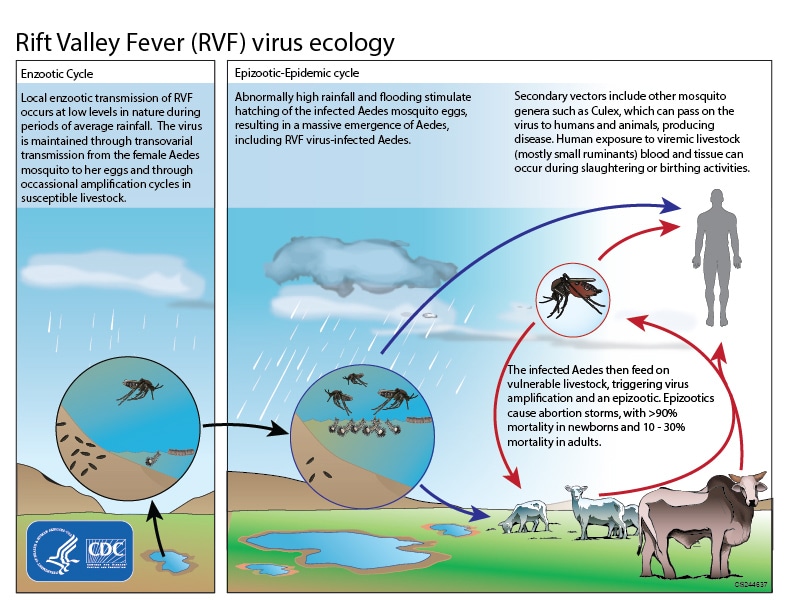
Rift Valley fever, a mosquito-borne disease, usually occurs in people following direct contact with infected animals or bites from infected mosquitoes. The virus spreads from the female mosquitoes to the eggs. As more mosquitoes hatch, the potential for the virus to spread directly to animals and people increases.
While the majority of people infected experience mild disease, a small proportion develops the severe hemorrhagic form, which can cause blindness, convulsions, encephalitis, and bleeding, and mortality rates of up to 50%.
CEPI and the University of Oxford are committed to enabling access to vaccine outputs developed through this partnership, which aligns with CEPI’s Equitable Access Policy.