Lyme Disease Vaccine Candidate Preps for Next Season
With Lyme disease cases steadily declining in the United States, the only vaccine candidate conducting late-stage clinical research announced very encouraging news today.
On September 3, 2025, Valneva SE reported positive immunogenicity and safety data from the ongoing Phase 2 study of Lyme disease vaccine candidate, VLA15.
The strong anamnestic immune response and favorable safety profile following a third booster dose were consistent with those reported after receiving previous annual booster doses, further demonstrating compatibility with the anticipated benefits of a yearly vaccination before each Lyme season, wrote the company.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release, "These latest data further reinforce the potential benefits of booster doses across all evaluated age groups.... as the disease continues to expand geographically, it remains a pressing unmet medical need affecting communities across the Northern Hemisphere."
"Each set of positive results moves us closer to the possibility of making this vaccine available to both adults and children living in Lyme-endemic areas."
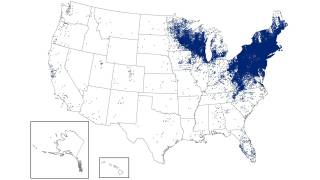
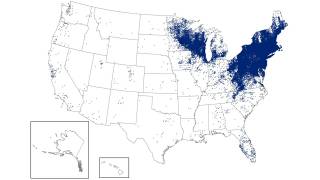
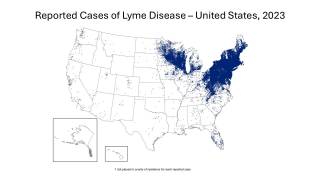
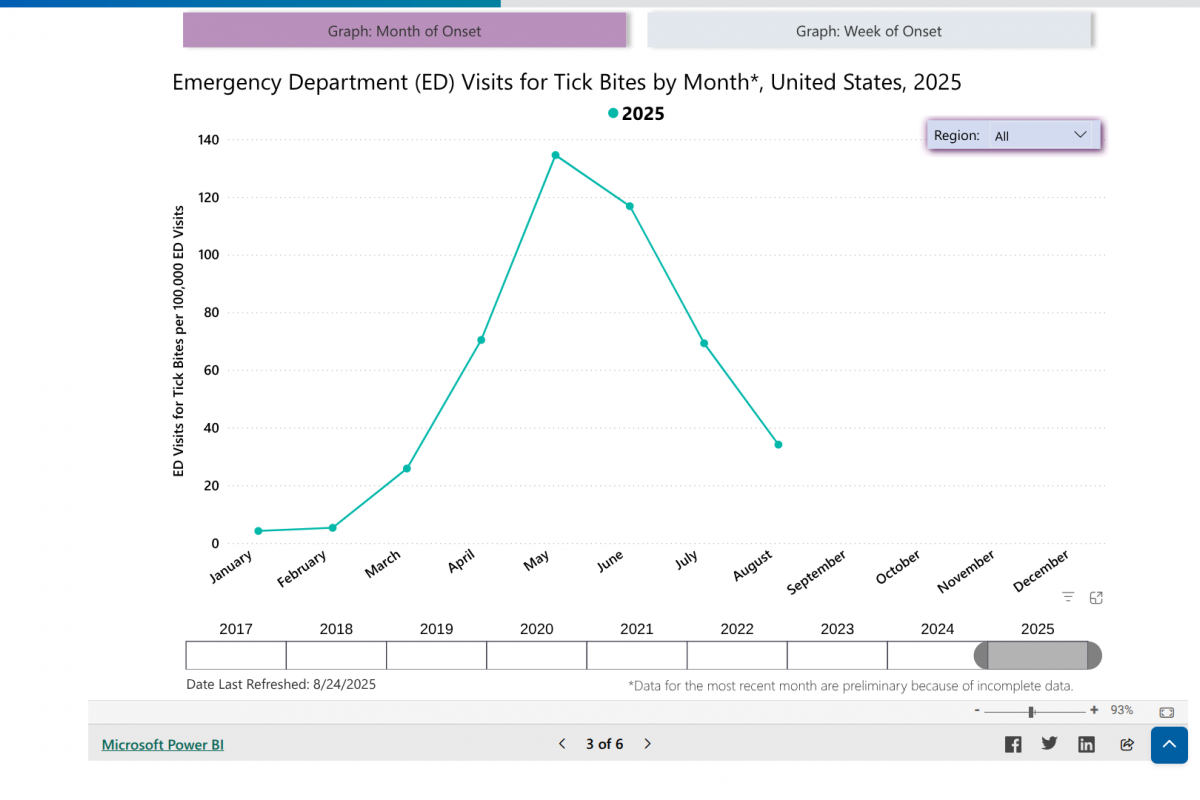
Lyme disease (Lyme borreliosis) is a bacterial disease transmitted to humans through the bite of infected ticks, initially detected in 1977 in Lyme, Connecticut, and now found in most northeastern states.
In the UK, ticks that carry Lyme disease are most active in the spring and summer. Approximately 4% of ticks in England and Wales are infected with Lyme disease.
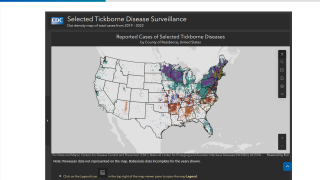
As of September 2025, the U.S. Centers for Disease Control and Prevention's Tick Bite Data Tracker displays case data and maps for the U.S.
Our Trust Standards: Medical Advisory Committee