Second Chikungunya Vaccine Posts Positive Phase 3 Results
Bavarian Nordic A/S today announced today the initial safety and immunogenicity results from a randomized, double-blind, placebo-controlled Phase 3 clinical trial of a virus-like particle (VLP)-based chikungunya virus (CHIKV) vaccine candidate CHIKV VLP (PXVX0317) in healthy adults.
The initial results up to Day 22 post-vaccination showed that CHIKV VLP was immunogenic in healthy adults ≥65 years of age, as demonstrated by a strong induction of CHIKV neutralizing antibodies in 87% of vaccinees with neutralizing antibody titres exceeding the threshold agreed with authorities as a marker of seroprotection, thus meeting the primary endpoints of the study.
Importantly, seroprotective neutralizing antibodies were also observed in most subjects (82%) at Day 15 post the single vaccination, demonstrating a fast onset of protection for the VLP-based CHIKV vaccine candidate.
This clinical trial will continue for a 6-month follow-up for both safety and immunogenicity.
Paul Chaplin, President and CEO of Bavarian Nordic, said in a press release on June 20, 2023, “While we still await the results from the second and larger Phase 3 study in adolescents and adults later this year, these highly encouraging results provide a high degree of confidence for our CHIKV vaccine program.”
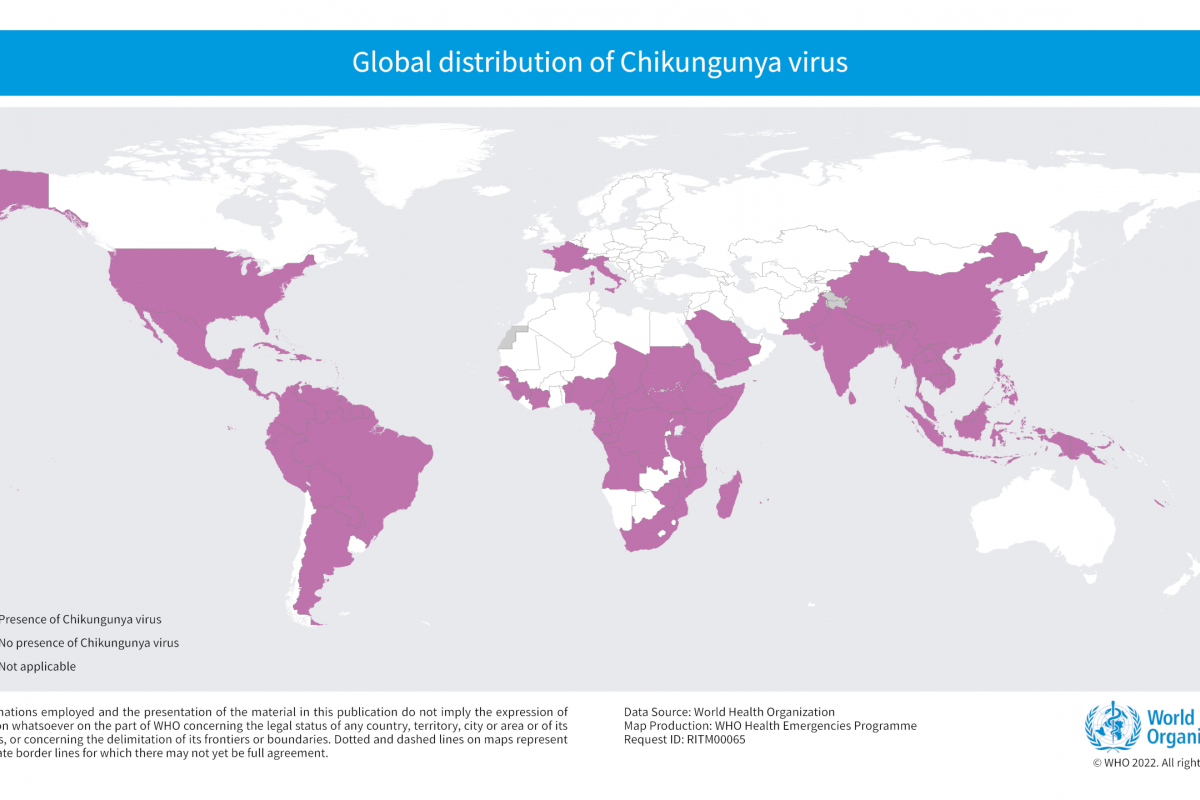
Chikungunya is a mosquito-borne viral disease caused by the Chikungunya virus (CHIKV), causing outbreaks in over 100 countries as of 2023.
From 2006–2021, 4,590 chikungunya cases in U.S. travelers were reported to the U.S. CDC.
While mortality is low, morbidity is high; nearly 50% of individuals with CHIKV disease have debilitating long-term symptoms that can intensify with age.
Additional Chikungunya vaccine news is posted by Precision Vaccinations.
Our Trust Standards: Medical Advisory Committee























