Lyme Disease Vaccine Candidate Approaches 2020 Finish-Line

A French biotech company announced the initiation of the second Phase 2 clinical trial for the development of its Lyme disease vaccine candidate VLA15.
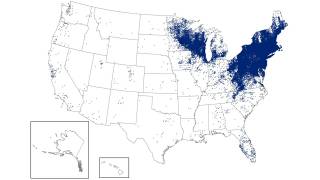
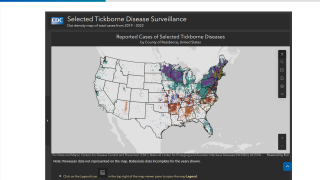
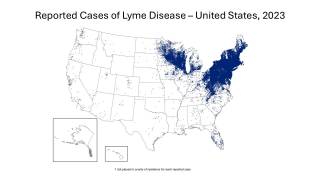
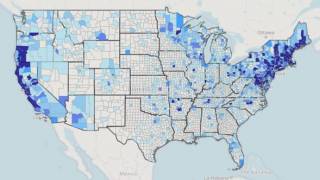
This is important news since the medical need for a Lyme disease preventive vaccine is steadily increasing as the disease footprint now reaches all 50 US states.
According to the U.S. Centers for Disease Control and Prevention (CDC), approximately 300,000 Americans are diagnosed with Lyme disease each year, with at least a further 200,000 cases in Europe.
The objectives for this VLA15-202 clinical study are to determine the optimal dosage level and vaccination schedule for use in Phase 3 pivotal field efficacy studies, based on immunogenicity and safety data.
The Phase 2 Clinical Study VLA15-202 is a randomized, observer-blind, placebo-controlled study of 250 subjects conducted at trial sites in the US.
Study results are expected in 2020.
Following the Run-In phase for Valneva’s first Phase 2 study VLA15-201, the two dosage levels (135µg and 180µg) have been selected for further development based on Data and Safety Monitoring Board clearance.
Wolfgang Bender, MD, Ph.D., Chief Medical Officer of Valneva, commented in a press release, “With higher dosage levels and the potential alternative vaccination schedule, our ultimate goal is to further optimize our vaccine candidate by targeting a high efficacy from the first Lyme season.”
The Phase 2 duration is expected to be approximately two years with initial data (primary endpoint) expected mid-2020.
Lyme Disease is a systemic infection caused by Borrelia bacteria transmitted to humans by infected Ixodes ticks. These ticks can occasionally transmit other tickborne diseases as well, says the CDC.
It is considered the most common vector-borne illness in the Northern Hemisphere. The CDC notes a 3.5-fold increase in vector-borne diseases in the U.S. from 2004-2016, with more than 76 percent of cases caused by tick-borne pathogens.
Left untreated, Lyme disease can disseminate and cause more serious complications affecting the joints, the heart or the nervous system.
Early symptoms of Lyme disease include a gradually expanding erythematous rash called Erythema migrans or more unspecific symptoms like fatigue, fever, headache, mild stiff neck, arthralgia or myalgia.
These symptoms are often overlooked or misinterpreted, says the CDC.
To address this need, the Infectious Diseases Society of America, American Academy of Neurology, and American Academy of Rheumatology proposed a draft on June 27th, 2019, for the prevention, diagnosis, and treatment of Lyme disease.
These draft guidelines are now accepting public comments through August 10, 2019. Those wishing to provide feedback can view the draft guidelines and submit their comments on the IDSA website.
Recent Lyme disease news:
Valneva’s vaccine candidate VLA15 was granted Fast Track designation by the U.S. Food and Drug Administration (FDA) in July 2017.
Our Trust Standards: Medical Advisory Committee