Pediatric Lyme Disease Vaccine Study Launched

A France-based specialty vaccine company announced its plans to accelerate pediatric development of the Lyme vaccine candidate, VLA15, in collaboration with Pfizer Inc.
Valneva SE stated in a press release the planned initiation of the study VLA15-221 would be in the first quarter of 2021, subject to regulatory approval.
If approved, it will be the first clinical study to enroll a pediatric population aged 5-17 years and compare the 3-dose vaccination schedule on month 0-2-6, with a reduced 2-dose schedule of month 0-6.
“This will be an important study that we anticipate will provide evidence that the vaccine can be used in the populations that are at risk of the devastating consequences of Lyme disease, using a simplified schedule,” stated Kathrin Jansen, Senior Vice President and Head of Pfizer Vaccine Research and Development.
VLA15-221 is planned as a randomized, observer-blind, placebo-controlled Phase 2 study. The study will currently include approximately 600 healthy participants (5-65 years of age) who will receive VLA15 at the dose of 180µg, which was selected based on recent data generated in the two ongoing Phase 2 studies.
VLA15-221 will also investigate a booster dose of VLA15, administered one year following the 6 Month dose.
This study will complement the ongoing Phase 2 studies VLA15-201 (July 2020) and VLA15-202 (October 2020).
All three Phase 2 trials are anticipated to support a Phase 3 pivotal efficacy trial, including all Lyme vaccine candidates' main target populations starting in 2022.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented, “We believe that including the pediatric population early on could provide support for the Phase 3 study to include all major target groups for our future Lyme vaccine candidate and may potentially support successful market access including respective recommendations.”
Valneva and Pfizer entered into a collaboration agreement in April 2020 to co-develop and commercialize VLA15.
VLA15 is the only active Lyme disease vaccine in clinical development today and covers six serotypes prevalent in North America and Europe. This investigational multivalent protein subunit vaccine targets the outer surface protein A (OspA) of Borrelia, an established action mechanism for a Lyme disease vaccine.
OspA is one of the most dominant surface proteins expressed by the bacteria when present in a tick. VLA15 has demonstrated promising immunogenicity and safety data in pre-clinical and clinical studies so far.
VLA15 will be tested as an alum-adjuvanted formulation and administered intramuscularly. The study will be conducted at sites located in areas where Lyme disease is endemic and will enroll volunteers with clear past infection with Borrelia burgdorferi, the bacteria that cause Lyme disease, and B. burgdorferi naïve volunteers, says the company.
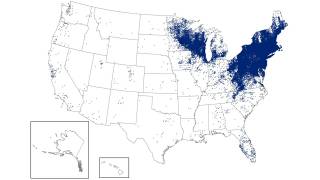
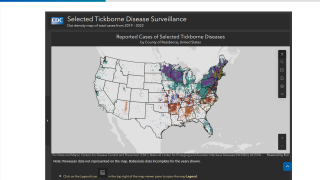
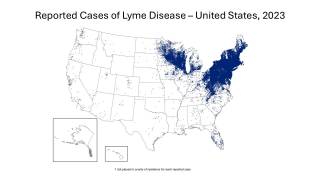
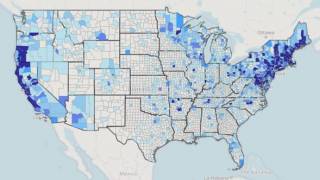
Lyme disease is a systemic infection caused by Borrelia bacteria burgdorferi sensu lato transmitted to humans by infected Ixodes ticks. It is considered the most common vector-borne illness in the Northern Hemisphere.
According to the U.S. Centers for Disease Control and Prevention (CDC), approximately 300,000 Americans are diagnosed with Lyme disease each year, with at least an additional 200,000 cases in Europe.
Early symptoms of Lyme disease are often overlooked or misinterpreted. Left untreated, the disease can disseminate and cause more serious complications affecting the joints (arthritis), the heart (carditis), or the nervous system.
For more information, visit Valneva.
PrecisionVaccinations publishes research-based vaccine development news.
Our Trust Standards: Medical Advisory Committee