Since late May 2025, France's overseas department of Mayotte has experienced a significant outbreak of the mosquito-transmitted Chikungunya virus throughout the island.
As of June 2, 2025, Public Health France reports a total of 560 confirmed cases of chikungunya recorded during this phase of the ORSEC arbovirus plan.
From a location perspective, there has been a persistent concentration of cases in the municipalities of Mamoudzou, Pamandzi, and Dzaoudzi.
These are Mayotte's first locally transmitted cases of chikungunya since the 2005–2006 outbreak, which resulted in approximately 7,300 cases.
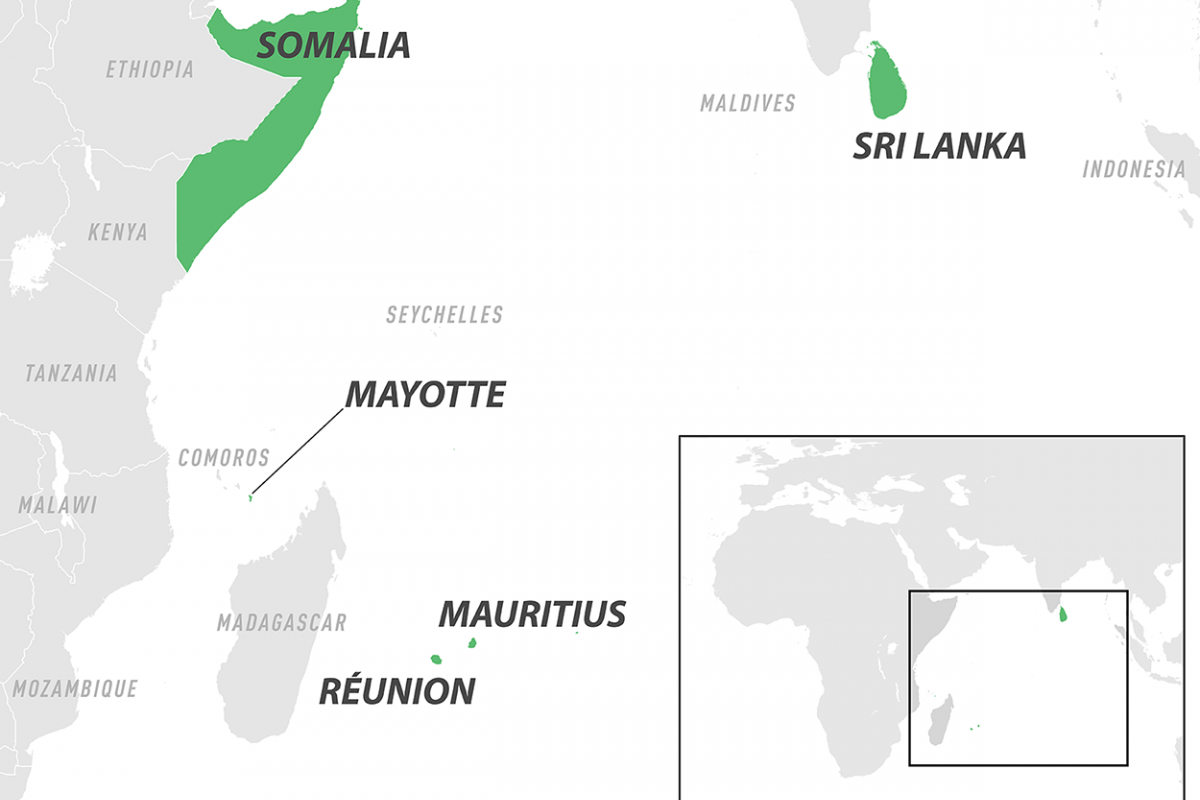
According to the World Health Organization (WHO), Chikungunya outbreaks have been documented on islands within and countries bordering the Indian and Pacific Oceans, such as Mauritius, Réunion, Somalia, and Sri Lanka, in 2025.
Public health response measures have included targeted vaccination efforts with Valneva SE's IXCHIQ® vaccine, the first approved monovalent, single-dose, live-attenuated vaccine.
While the WHO states that no measures related to international traffic and trade are warranted at this time, the U.S. CDC has updated its information to a Level 2 - Practice Enhanced Precautions, Travel Health Advisory.
The CDC recommends vaccination for travelers visiting an area with a chikungunya outbreak. Furthermore, if you are pregnant, consider reconsidering travel to the affected areas, particularly if you are nearing the delivery of your baby. Mothers infected around the time of delivery can pass the virus to their baby before or during delivery.
In addition to chikungunya, another mosquito-transmitted disease has been spreading on Mayotte. The circulation of dengue fever on the island has reached 21 confirmed cases since the beginning of 2025.
Before visiting Mayotte in June 2025, the CDC recommends consulting with a travel vaccine specialist to discuss immunization options.