Oral Pill Norovirus Vaccine Candidate Posts Positive Phase 2b Challenge Study Results
With the summer of 2025 cruise ship season getting underway, many passengers are seeking access to a norovirus preventive vaccine.
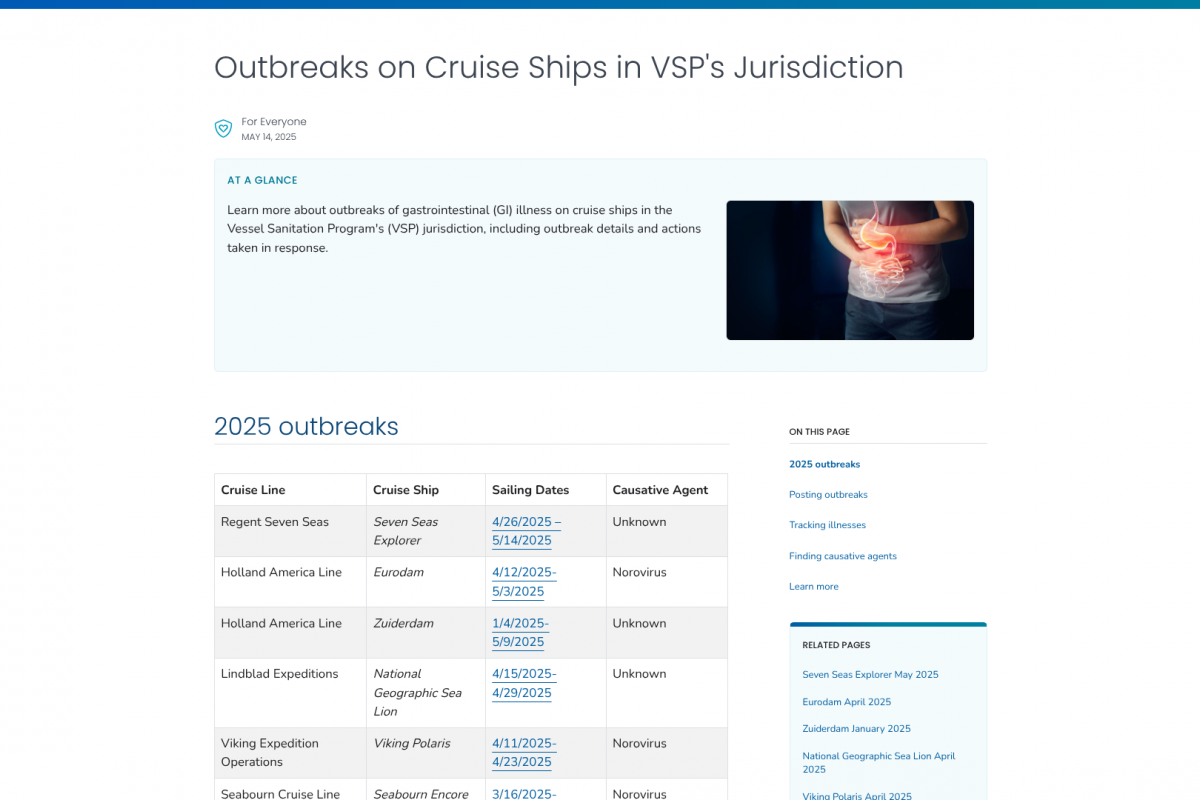
As of May 14, 2025, the U.S. CDC's Vessel Sanitation Program had reported 17 gastrointestinal (GI) illnesses on cruise ships this year, with over ten classified as norovirus outbreaks, and others still under investigation.
This CDC data compares with 18 GI outbreaks in all of 2024 and just 14 in 2023.
While norovirus vaccine research has been previously unsuccessful, one oral vaccine has published positive news.
Vaxart, Inc. recently announced the publication of complete data from a Phase 2b challenge study of its first-generation oral pill norovirus vaccine candidate.
This study measured safety, efficacy against infection and symptomatic disease, as well as viral shedding.
Additionally, a machine learning analysis identified statistically significant correlates of protection, which will be incorporated into the development of Vaxart’s second-generation norovirus vaccine candidate.
“Challenge studies provide unique opportunities to identify correlates of protection that can be used to predict vaccine efficacy and support vaccine development,” said James F. Cummings, MD, Chief Medical Officer at Vaxart, in a press release.
“The application of machine learning approaches to the complete data from the Phase 2b challenge study of our first-generation oral pill norovirus vaccine candidate identified two such correlates, functional serum blocking antibody and fecal IgA."
"Evaluation of these endpoints will help inform our understanding and provide an early read on the potential efficacy profile of our second-generation norovirus vaccine candidate as it advances through clinical development."
The single-center, double-blinded Phase 2b challenge study enrolled 165 healthy adults, who were randomized 1:1 to receive Vaxart’s monovalent oral pill vaccine candidate targeting the norovirus GI.1 genotype or placebo. Four weeks after vaccination, subjects were challenged with GI.1 norovirus. The primary objective of the study was to determine the efficacy of the vaccine against norovirus infection and norovirus gastroenteritis (NVG) following gastrointestinal (GI) infection.1 NV challenge.
Secondary objectives were to assess the safety and tolerability of the vaccine candidate. The ability of the vaccine candidate to modify disease severity, the quantity and duration of norovirus shedding, and a set of immunogenicity parameters was also quantified. The primary efficacy endpoints were the proportion of participants showing evidence of NVG, a composite endpoint defined as meeting one or more definitions for acute gastroenteritis and a positive norovirus infection, and the norovirus infection itself.
Key findings from the study include the vaccine was immunogenic and protected against norovirus infection, with a 30% relative reduction for the vaccine group compared with placebo (p=0.003); The vaccine group had a lower incidence of norovirus gastroenteritis (21% relative reduction), but was not statistically different (p=0.178); The vaccine significantly increased serum IgA, IgG, norovirus-blocking antibodies, and antibody-secreting cells (p<0.001 for all endpoints). The vaccine stimulated mucosal-homing B cells and significantly increased norovirus-specific antibodies in saliva, nasal lining fluid, and the intestine.
Steven Lo, Chief Executive Officer of Vaxart, added, “The initiation of the Phase 1 clinical trial comparing our first- and second-generation norovirus vaccine candidates is a key step toward this important goal."
"The Phase 2 challenge study published today for our first-generation norovirus candidate supports our oral pill norovirus vaccine approach, and the preclinical data we have generated to date support our view that our second-generation candidate has the potential to provide improved immunogenicity and protection.”
As of May 30, 3035, the CDC has not recommended any norovirus vaccine.
Our Trust Standards: Medical Advisory Committee






