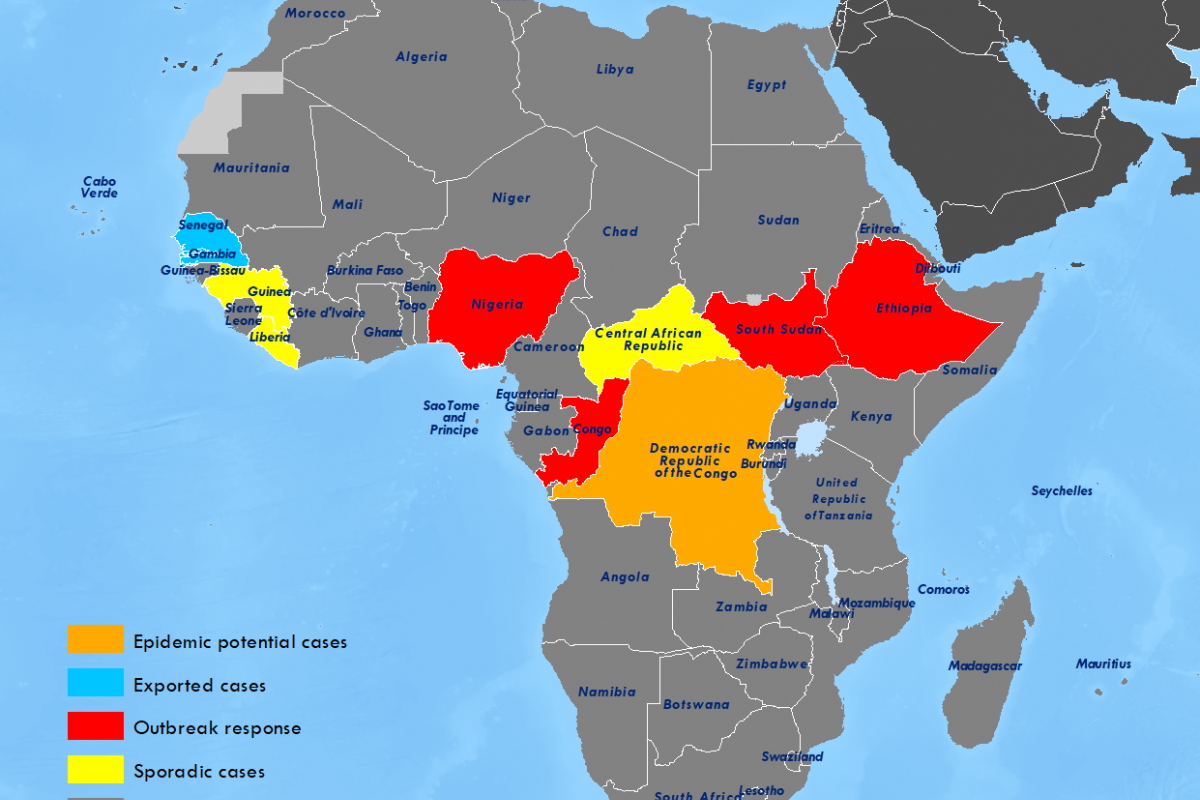
Few disease outbreaks have been as deeply traumatic as the 2014 Ebola outbreak in West Africa. By the time the outbreak stopped in 2016, nearly 30,000 had been infected, and 11,000 people had died.
With no vaccine available at the outbreak's start, the affected countries, Guinea, Sierra Leone, and Liberia, were unprepared to respond.
This unfortunate situation has changed with the approval of Ebola vaccines and therapeutics.
Launched in January 2021, the International Coordinating Group on Vaccine Provision, which includes the World Health Organization, UNICEF, and others, now coordinates a stockpile.
It is a Gavi-funded, globally managed reserve of Merck's ERVEBO® (rVSV-ZEBOV) vaccine that ensures rapid, equitable access to life-saving immunisation during outbreaks.
The vaccine stockpile in Switzerland is maintained at a target level of 500,000 ERVEBO doses, as the WHO’s Strategic Advisory Group of Experts on Immunization recommends.
A challenge in maintaining a stockpile is ensuring that doses are always available and do not expire.
“All Ebola outbreaks that have occurred since we had a stockpile were quickly stopped – thanks to the vaccines and rapid other response measures,” said Allyson Russell, an epidemiologist and senior programme manager in Gavi’s High Impact Outbreaks team, in a April 30, 2025 news release.
In the United States, ERVEBO® is approved by the Food and Drug Administration for preventing disease caused by the Zaire Ebola virus in individuals 12 months of age and older as a single-dose administration.
Zaire Ebola vaccines aren't effective against the other three orthoebolaviruses that cause severe disease, including the Sudan virus.
Since there are no well-controlled studies of ERVEBO in pregnancy, the U.S. CDC says, 'The risk of exposure to Ebola should be weighed against potential vaccine-related risk during pregnancy based on individual informed decisions.'
As of May 12, 2025, access to Ebola vaccines in the U.S. is restricted.