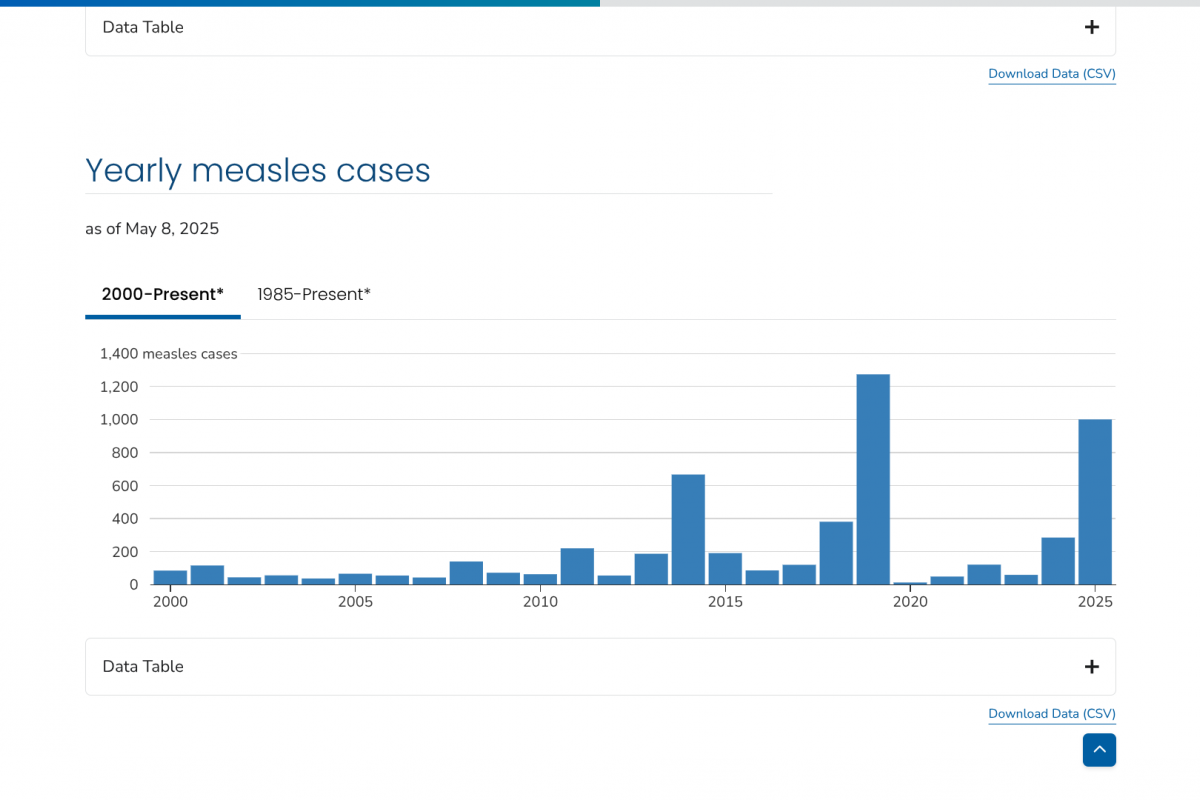
Since the first anthrax fatality in decades was reported in the Kingdom of Thailand in early May 2025, substantial confusion regarding a potential outbreak has been reported on social media.
To clarify the situation as of May 8, 2025, Dr. Pricha Worahan, Public Health Doctor of Nakhon Phanom Province, revealed that the anthrax situation in Mukdahan Province has been continuously monitored.
As for the latest disease situation, there are no confirmed anthrax patients. Anthrax is a zoonotic disease caused by the bacterium Bacillus anthracis.
There was one suspected anthrax patient found, a 55-year-old female farmer who denied any underlying diseases. She had a blister on her right arm that broke in the middle, making the wound black like a cigarette burn.
The doctor initially diagnosed anthrax as suspected, so he collected samples of the blister from her skin and Hemoculture to send to the laboratory of Nakhon Phanom Hospital and Bamrasnaradura Institute. No one with symptoms that fit the definition of searching for contacts of the disease was found.
However, two people who had contact with the infected person did not show any symptoms, and animals in the area did not show any abnormalities or die of unknown causes.
There are no reports of human-to-human transmission of the disease.
The most common vectors are cows, buffalo, goats, and sheep. Infected animals will have fever, be lethargic, not eat, get sick for no apparent reason, and die. Most people get infected by direct contact with infected animals, such as butchering meat, consuming raw or undercooked meat, or coming into contact with animal skins or fur that contain spores of the disease.
The germs contaminate the area where an animal is sick or dies and can remain there for months or years. Patients will have fever, body aches, cough, difficulty breathing, a blue face, and die from respiratory failure.
According to the U.S. CDC, anthrax is rare in the United States, but outbreaks do happen in wild and domestic grazing animals such as cattle or deer. In the U.S., veterinarians recommend yearly livestock vaccination in areas where animals have had anthrax. Additionally, anthrax vaccines for people are available in 2025.
Recently, South Korea approved BARYTHRAX, the world's first recombinant anthrax vaccine, jointly developed by GC Biopharma and the Korea Disease Control and Prevention Agency.
BARYTHRAX utilizes protective antigen (PA) proteins produced through genetic recombination techniques. With an anthrax infection, PA is a gateway for 2 Bacillus anthracis toxins, lethal factor and edema factor, to enter host cells. BARYTHRAX vaccination can train and stimulate an immune response to neutralize anthrax by utilizing PA proteins.