World's First Recombinant Anthrax Vaccine Approved in Korea
The South Korean Ministry of Food and Drug Safety recently approved BARYTHRAX, an anthrax vaccine jointly developed by GC Biopharma and the Korea Disease Control and Prevention Agency.
Traditional anthrax vaccines are made by attenuating Bacillus anthracis or culturing non-pathogenic Bacillus anthracis, which may contain residual toxin components. BARYTHRAX removes this risk and improves vaccine safety.
BARYTHRAX utilizes protective antigen (PA) proteins produced through genetic recombination techniques. With an anthrax infection, PA is a gateway for 2 Bacillus anthracis toxins, lethal factor and edema factor, to enter host cells.
BARYTHRAX vaccination can train and stimulate an immune response to neutralize anthrax by utilizing PA proteins.
Eun-chul Huh, President and CEO of GC Biopharma, commented in a press release on April 9, 2025, "This achievement underscores our commitment to localizing critical medicines for public health and national security. GC Biopharma will continue leading efforts to ensure stable supplies of essential medical products, as we have been doing with other vaccines and blood products since our founding."
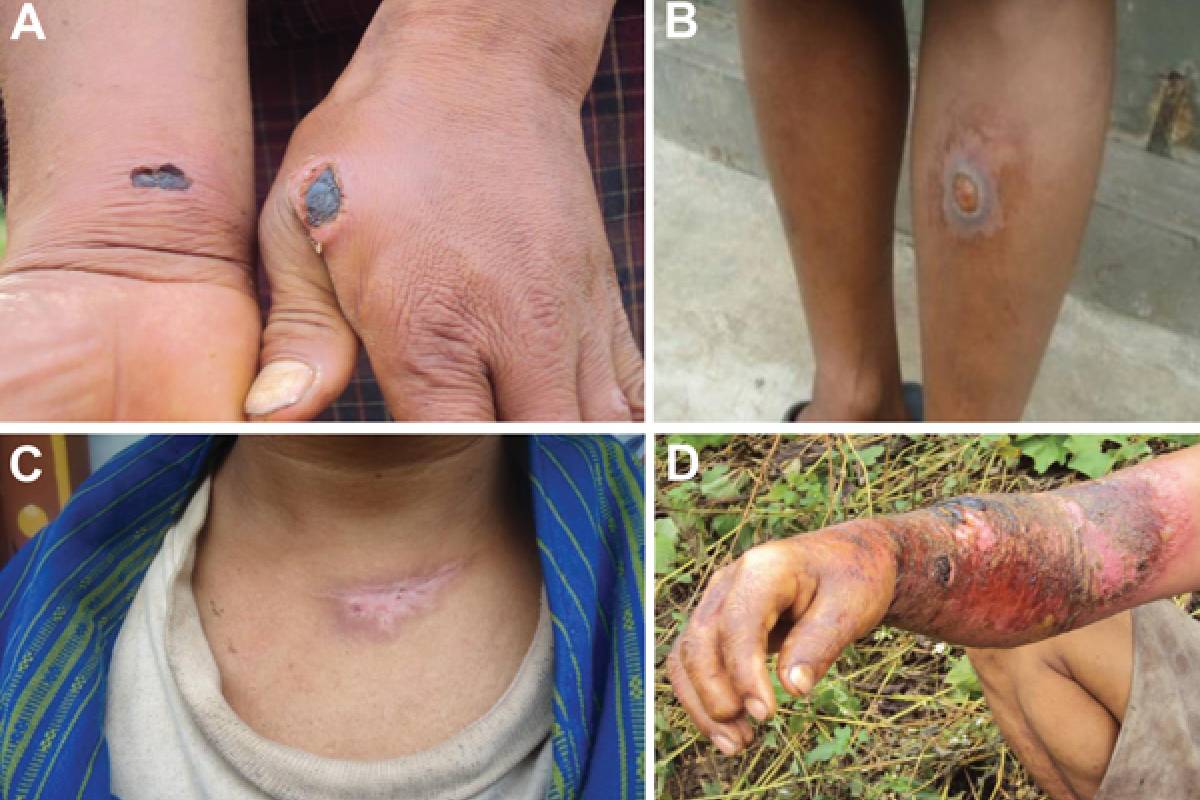
Anthrax, caused by Bacillus anthracis, is a class-1 infectious disease capable of surviving extreme conditions and spreading easily through airborne transmission. If untreated, its fatality rate can reach up to 97%, making it a significant threat as a potential biological weapon.
The MFDS's approval, supported by GC Biopharma's production capacity, will pave the way for the company to supply Korea's essential anthrax vaccine reserve.
In the United States, very few people get anthrax from infected animals or contaminated animal products. The U.S. CDC says The type of illness a person develops depends on how anthrax enters the body: through the skin, lungs, or gastrointestinal system.
Getting a vaccine or taking certain antibiotics after exposure to anthrax can help prevent illness.
As of April 9, 2025, the U.S. FDA has approved anthrax vaccines for those at risk of exposure to anthrax bacteria.
For example, CYFENDUS™ is a combination of BioThrax® (anthrax vaccine adsorbed) and CPG 7909, a synthetic short DNA sequence as a vaccine adjuvant.
Our Trust Standards: Medical Advisory Committee
