Vaccination Provides 30% Probability of Preventing Gonorrhea

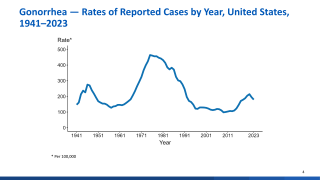
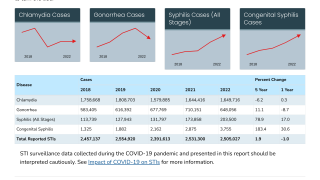
With about 8 million cases annually, gonorrhea is one of the most common sexually transmitted infections, without a U.S. FDA-approved vaccine available.
However, another study has concluded that leveraging an existing vaccine provides some cross-protection against N. gonorrhoeae.
Published by the journal Vaccine (Volume 56, 22 May 2025, 127180), this study evaluates the potential effectiveness of outer membrane vesicle (OMV)-based meningococcal B vaccines in preventing this sexually transmitted infection.
A recent review and meta-analysis, which included nine studies, found a pooled vaccine effectiveness of 30% when a 4CMenB vaccine was administered.
The University of West Attica researchers concluded, "While randomized clinical trials are necessary, the findings of this systematic review and meta-analysis highlight the potential effectiveness of OMV-based vaccines in preventing gonorrhea."
This new research, along with previous studies, suggests that the four-component serogroup B meningococcal vaccine (4CMenB) and other OMV-based MenB vaccines might offer such protection against this disease.
4CMenB vaccinations are offered at many community pharmacies in the United States in 2025.
Our Trust Standards: Medical Advisory Committee