The U.S. The Transportation Security Administration (TSA) has deployed various customer service resources and digital technologies to assist travelers in expediting the airport screening process.
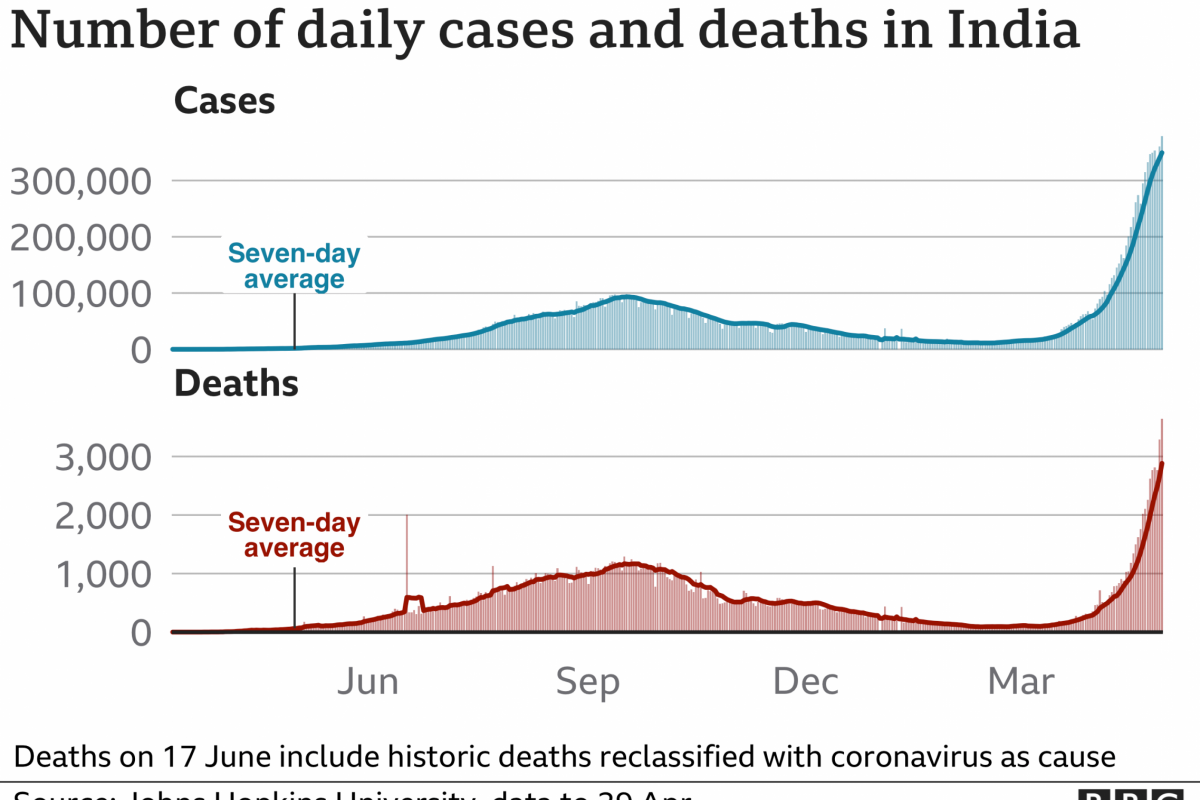
These TSA services enable about 2.2 million air travelers to pass through airport security more effectively in April 2023.
According to the TSA's activity report on April 19, 2023, the number of air travelers recently exceeded 2019, before the pandemic curtailed travel.
And if you need a helping hand during screening, all you have to do is ask TSA.
Air travelers requiring special accommodations or concerned about the airport security screening process can ask TSA staff for a passenger support specialist, who provides on–the–spot assistance during the security screening process.
Furthermore, those with questions or concerns about traveling with a disability, medical condition, or other exceptional circumstances can contact the TSA Cares helpline 72 hours before traveling.
Travelers may provide the officer with the TSA notification card or other medical documentation to describe their condition.
TSA Cares answers questions about screening policies, procedures, and what to expect at the security checkpoint.
TSA Cares can also assist passengers with medical conditions and injured service members, veterans, and wounded warriors.
The TSA's layered approach to security gets air passengers quickly and safely to their destination.
For example, in Houston, Texas, Hobby Airport (HOU) TSA Security Wait Times are digitally displayed.
Before heading to the airport, be sure to check HOU Arrival and Departure Delays, or if you know your specific flight, use iFly's Flight Tracker.
TSA's screening procedures are intended to prevent prohibited items and other threats to transportation security from entering the sterile area of the airport