Cholera Vaccines
Cholera Vaccines 2026
The U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), the World Health Organization (WHO), and the U.K. National Health Service (NHS) recommend oral cholera vaccines (OCVs) for travelers visiting countries experiencing outbreaks. As of December 2025, the World Health Organization (WHO) has prequalified Vaxchora®, Dukoral®, Shanchol™, Euvichol®, and Euvichol-S OCVs.
In January 2025, the WHO stated that OCV production had reached 6.2 million doses. However, as of May 2025, the OCV stockpile consisted of 5.7 million doses, below the minimum emergency threshold of 5 million. The WHO says that all OCVs require two vaccine doses for complete protection for up to three years, while a single dose provides short-term protection. GAVI reported in 2025 that, during the current cholera outbreak, only one OCV dose course had been implemented in reactive vaccination campaigns.
Dukoral® is administered with a buffer solution that requires 150 ml of clean water for adults. It can be administered to all individuals aged two and above.
Vaxchora® (lyophilized CVD 103-HgR) is a single-dose, oral vaccine that the U.S. FDA approved in June 2016. The safety and effectiveness of VAXCHORA have not been established in individuals with immunocompromised conditions. In August 2023, the U.S. Centers for Disease Control and Prevention (CDC) published "Cholera Vaccine: Recommendations," highlighting CVD 103-HgR Vaxchora for travelers going to areas of active toxigenic Vibrio cholerae O1 transmission.
Shanchol™ and Euvichol® are essentially the same vaccines produced by two different manufacturers. Euvichol-Plus® is a simplified formulation of the existing inactivated OCV jointly developed by Eubiologics and the International Vaccine Institute for cholera prevention. Euvichol®-S improves productivity by approximately 40% over Euvichol-Plus®. In January 2025, a batch of 948,500 doses of Euvichol-S arrived in Angola.
HILLCHOL® (BBV131) is a novel single-strain OCV developed by Bharat Biotech International Limited under Hilleman Laboratories license and funded by Merck and Wellcome Trust. It is a two-dose vaccine that BBIL states needs to be administered orally on Day 0 and Day 14. HILLCHOL is suitable for individuals over one year old and is available in mono- or multidose formats.
Cholera Vaccine Supply
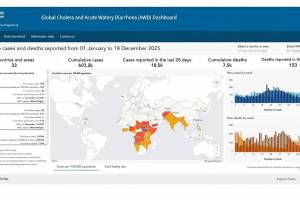
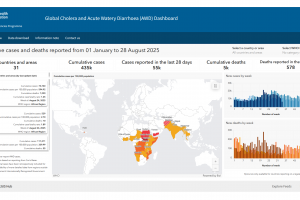
The International Coordinating Group (ICG) on Vaccine Provision has managed and coordinated the provision of OCV supplies and vaccines since 2013. Since the ICG's establishment in 1997, the WHO, UNICEF, and Médecins sans Frontières have facilitated the distribution of over 73 million doses of OCV to 23 countries. The OCV vaccine dashboard was updated for 2025. In May 2025, the average stockpile of Oral Cholera Vaccine stabilized at 5.7 million doses, the sixth consecutive month above five million. The WHO noted that the increased OCV production has yet to meet growing global needs as demand exceeds supply.
Cholera Outbreaks
Cholera outbreaks continued in 26 countries as of 2025.
Cholera Vaccine News
June 17, 2025 - The WHO published a Multi-country cholera outbreak, External Situation Report #27.
December 18, 2024 - The WHO reported that a persistent shortage of OCVs continues to hinder efforts to control cholera outbreaks and respond promptly to the spread of the disease.