Antimalarial Treatments
Antimalarial Treatment 2025
The U.S. Centers for Disease Control and Prevention (CDC) Algorithm for the Diagnosis and Management of Malaria outlines recommended steps for treating malaria patients. The CDC's Yellow Book outlines various treatment options for Malaria. Antimalarial treatment must be initiated immediately upon diagnosis of Malaria, according to the CDC's Malaria Diagnosis and Treatment. Furthermore, the CDC's Malaria Treatment Tables guide malaria treatment, and options for treating pregnant women are presented in the Alternatives for Pregnant Women. However, presumptive treatment should be reserved for extreme circumstances, such as when there is clinical suspicion of severe disease in a setting where prompt laboratory diagnosis is unavailable.
The World Health Organization's (WHO) recommendations on malaria elimination strategies may include mass drug administration, testing, treatment, or prevention of mass relapse. These strategies are generally not recommended in elimination settings unless there is local malaria transmission. The WHO published a Q&A developed by the Global Malaria Programme in collaboration with the Pan American Health Organization (PAHO).
Malaria Medications
On November 12, 2025, Novartis announced the Phase III trial for KLU156 (ganaplacide/lumefantrine, or GanLum), which meets the primary endpoint of non-inferiority to standard of care Coartem® (artemether-lumefantrine); Demonstrates PCR-corrected cure rate of 97.4% based on estimand method, equating to 99.2% under conventional per protocol analysis; the Novel ingredient in GanLum, ganaplacide, has an entirely new mechanism of action. If approved, GanLum would represent the first significant innovation in malaria treatment since 1999, with the potential to kill drug-resistant parasites and block transmission.
On July 22, 2025, the New England Journal of Medicine published a study that found ivermectin, an antiparasitic drug, reduced the incidence of Malaria by 26%. A 2019 study concluded similar benefits.
On July 8, 2025, Novartis announced that Swissmedic had approved Coartem® Baby (Riamet® Baby) as the first malaria medicine for newborns and young infants. The new treatment was developed in collaboration with Medicines for Malaria Venture to treat the potentially deadly mosquito-borne disease.
As of July 2025, four treatment options are available for P. falciparum infections acquired in areas with chloroquine resistance, specifically for adolescents and adults. These include artemether-lumefantriCoartemtem®), the preferred option if readily available, and atovaquone-proguanil (Malarone), according to the CDC. On June 20, 2023, the BMC Malaria Journal published a perspective article titled "ve: Real-life effectiveness of antimalarial treatment regime". Options for the treatment of pregnant women are presented in the Alternatives for Pregnant Women.
Tafenoquine is aKODA™), an 8-aminoquinoline, an antimalarial approved in the U.S., and it targets the liver stage of P. vivax malaria. When combined with chloroquine, tafenoquine provides a radical cure for the treatment of both the disease's blood and liver stages.
Novartis and Medicines for Malaria Venture announced on November 23, 2022, their decision to proceed with a Phase 3 study for a novel ganaplicide/lumefantrine-SDF combination in adults and children with Malaria. Ganaplacide is a novel agent with a new mechanism of action combined with a new formulation of lumefantrine optimized for once-daily dosing. Pyramax® (pyronaridine-artesunate) was included in the WHO's list of pre-qualified medicines in 2012 and the WHO's Essential Medicines Lists for adults and children in 2017. Pyramax subsequently underwent a positive review by the WHO's Advisory Committee on the Safety of Medicinal Products in 2019, including interim data from the CANTAM study.
Ocean Biomedical announces the discovery of a new therapeutic approach that could lead to the development of a novel class of antimalarials. Promising results from the malaria program include a monoclonal antibody that kills 94%-99% of malaria parasites in culture, as well as a small-molecule drug that kills 100% of parasites at low nanomolar concentrations. On October 24, 2023, Chief Scientist Jonathan Kurtis, MD, PhD, received the prestigious Falk Medical Research Trust Transformational Award of $1 million to advance a new class of antimalarial drugs, specifically targeting Candida.
Global Health Innovative Technology Fund announced on December 14, 2023, that it would invest approximately $3.3 million in a global, multicenter Phase III clinical trial project led by Fosun Pharma for a triple artemisinin combination drug (Artemether-Lumefantrine-Amodiaquine fixed-dose formulation) against Malaria, aiming to accelerate the development and commercialization of this new drug combination.
Insight Partners publishes a new research report on the global malaria treatment market, which is projected to grow at a CAGR of 4.9% from 2022 to 2028.
Malaria Monoclonal Antibody
Artesunate for Injection™ was FDA-approved in May 2020 and is indicated for infants, children, adults, and pregnant women with severe Malaria or those unable to tolerate oral antimalarials. Available in vials of 110mg, Artesunate for Injection is dosed at 2.4 mg/kg given intravenously at 0, 12, and 24 hours, then daily for up to seven days. If after 24 hours of Artesunate, the percent parasitemia is ≤ 1% and the patient can tolerate oral medications, the patient can be switched to an antimalarial regimen.
The Lancet Infectious Diseases published results from an NIAID phase 1 clinical study on January 25, 2023, that found the antimalarial monoclonal antibody CIS43LS conferred high protection against parasitemia at doses of 20 mg/kg or 40 mg/kg administered intravenously fo,llowed by controlled human malaria infection, providing evidence that this approach might be helpful to prevent Malaria across several clinical use cases. The NEJM published an Original Article on October 31, 2022: Safety and Efficacy of a Monoclonal Antibody against Malaria in Mali. CONCLUSIONS - CIS43LS was protected against P. falciparum infection in a phase 2 clinical trial over a 6-month malaria season without evident safety concerns. In a related Editorial by Umberto D'Alessandro, M.D., Ph.D., currently available interventions for malaria control are unlikely to achieve the vision of a malaria-free world. And the NEJM published Aan ORIGINAL ARTICLE on August 4, 2022, anw-Dose Subcutaneous or Intravenous Monoclonal Antibody to Prevent Malaria. On October 18, 2022, the peer-reviewed journal Cell published results from a phase 1 clinical trial that concluded that S, a potent and safe antimalarial monoclonal antibody, demonstrated 88% protective efficacy against infection in healthy adults.
Malaria Testing
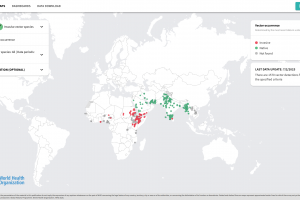
Malaria tests can detect parasites in a person's blood.
Malaria Netting
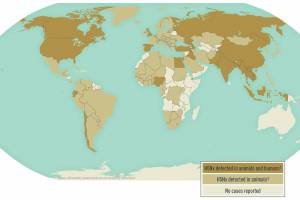
On March 14, 2023, the WHO recommended a new class of long-lasting insecticide-treated nets (LLINs) that combine insecticide mixtures with different modes of action. The Lancet published results from a study in January 223 that found chlorfenapyr-pyrethroid LLINs provided greater protection from Malaria than pyrethroid-only LLINs in an area with pyrethroid-resistant mosquitoes. Pyriproxyfen-pyrethroid LLINs conferred protection similar to pyrethroid-only LLINs. A study published by the BMJ Global Health Journal suggests that LLINs reduced the incidence in the first year. However, of the 88 malaria-endemic countries that provided data for 2010–2020, 78 did not report detecting resistance to at least one insecticide class reported by WHO in December 2022.
Malaria Vaccines
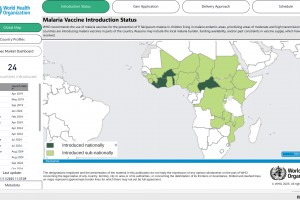
Malaria vaccines are approved for use in 2025.