Universal Flu Shot Clinical Trial Launches
A clinical-stage biotechnology company recently announced that the first participants have been dosed in the company's Phase 1A clinical trial evaluating Centi-Flu 01, a pan-influenza universal flu vaccine.
According to Centivax, the Phase 1A represents a key milestone toward a new kind of flu vaccine designed to provide broader, more reliable protection than standard seasonal vaccines, protecting against currently circulating strains, future strains, and pandemic strains.
The first data from this studyis expected within the year.
Unlike conventional seasonal influenza vaccines, which must be reformulated annually to attempt to match predicted circulating strains, Centi-Flu 01 is designed to focus both antibody and cellular immune responses on conserved regions of the influenza virus that cannot mutate and are shared across strains and distance subtypes.
This approach aims to generate broad, consistent, and durable immunity against both seasonal and pandemic influenza.
Sawsan Youssef, PhD, founder and Chief Science Officer of Centivax, stated in a press release on February 12, 2026, "A universal influenza vaccine allows us to be proactive—moving from annual guesswork to predictable, durable response."
In addition to safety, the study will evaluate efficacy based on established correlates of protection, using the gold-standard hemagglutination inhibition (HAI) assay against a panel of more than twenty flu strains—including currently circulating strains, historical mismatch strains, seasonal guidance strains, and pandemic strains—in a direct head-to-head comparison with existing standard-of-care flu vaccines.
Because the HAI assay is the same correlate-of-protection used to license seasonal flu vaccines, positive data will provide a clear benchmark demonstrating the candidate's ability to deliver broad protection with a single vaccine.
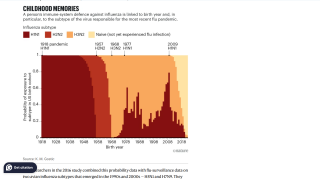
This type of innovative flu shot is essential because current vaccines are suboptimal at preventing virus transmission.
The 2024-2025 seasonal flu vaccine (trivalent formulation provided moderate protection in the United States, according to interim estimates from the U.S. CDC. Seasonal influenza effectiveness estimates among children and adolescents was 32%, 59%, and 60% in outpatient settings.
Our Trust Standards: Medical Advisory Committee