Baculovirus Platform Prepares to Protect People From Bird Flu
The world's largest vaccine manufacturer, Serum Institute of India (SII) Pvt. Ltd., is enhancing its pandemic response preparedness by utilizing a baculovirus vaccine platform specifically designed to target H5N1 bird flu.
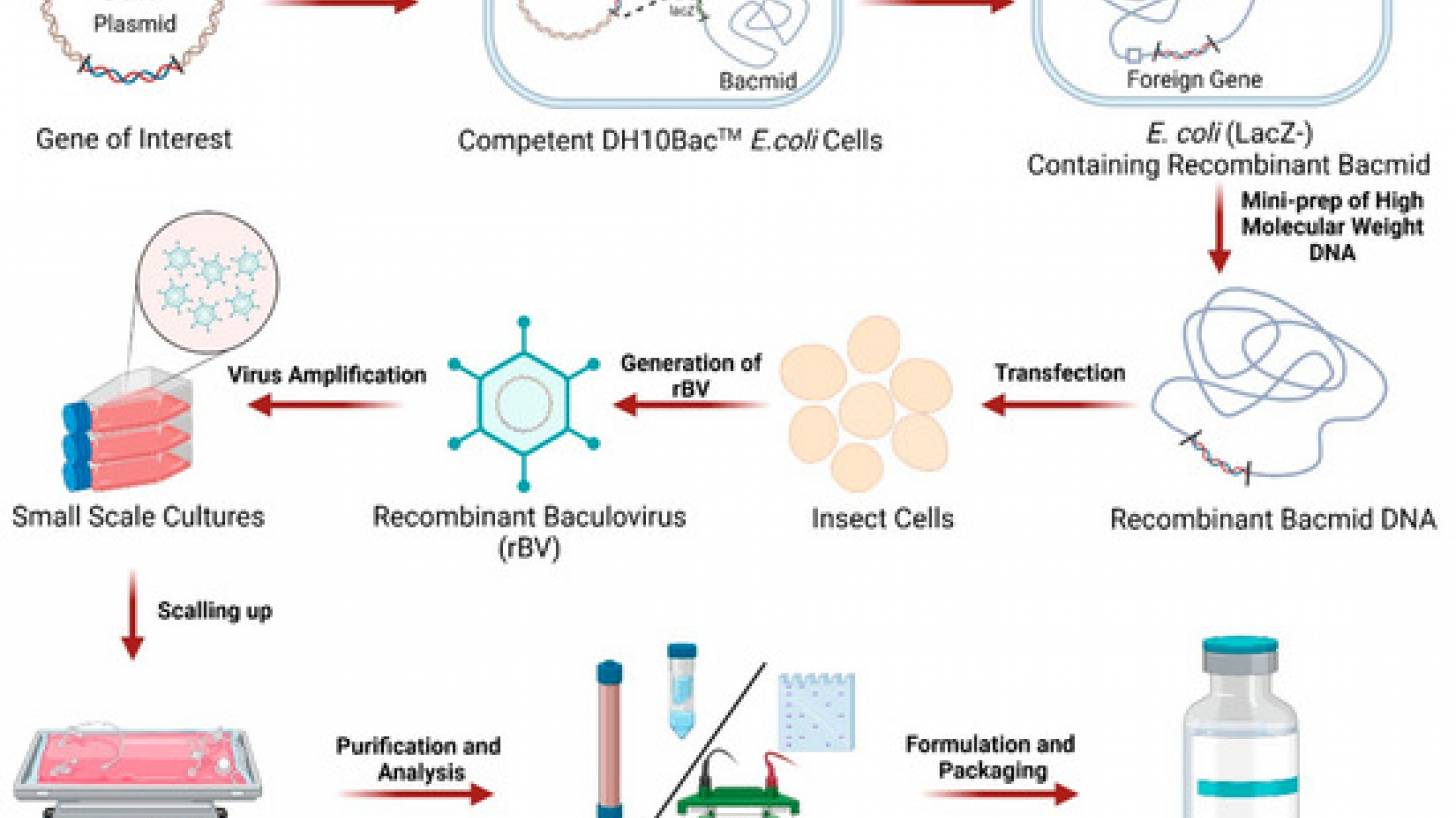
According to SSI, Baculovirus-based vaccines use a harmless insect virus that has been genetically modified to produce an antigen of a given virus. As they can be quickly created once a viral sequence is available, they are an ideal candidate for faster responses against potential pandemic diseases.
Supported by CEPI funding of up to $16.4 million, SII will use its validated baculovirus-based platform to produce and compare two H5 antigens for a recombinant protein vaccine: a wild-type and an AI-optimized, broad-spectrum H5 antigen designed by scientists at Houston Methodist Research Institute.
The broad-spectrum approach is designed to elicit immune responses across multiple strains of H5 viruses, rather than just one, making it particularly suited for use in unpredictable outbreak situations.
The collaboration will ultimately pressure-test the baculovirus platform's ability to quickly produce new antigens against H5 viruses, simulating a fast response to a future pandemic threat. The work will also serve as proof of concept for using artificial intelligence (AI) to design vaccine antigens capable of inducing broadly protective immunity.
"This new project—which deepens our collaboration with SII, one of CEPI's preferred vaccine manufacturing partners—is designed to power up global readiness to tackle pandemic threats, from early-stage vaccine development through to global manufacture and supply," said Dr Richard Hatchett, CEO of CEPI, in a press release.
"With a potential pandemic influenza vaccine candidate already in development on a validated platform, and with a vaccine manufacturing juggernaut ready to go, the world's disease defenses will be poised to respond swiftly with new vaccines, potentially in 100 days, should a flu virus erupt into a potentially deadly and fast-spreading human pandemic."
This initiative aligns with CEPI's 100 Days Mission—a goal embraced by leaders of the G7 and G20 to accelerate vaccine development to within 100 days of identifying a pandemic threat, such as Disease X.
In 2024, SII joined CEPI's vaccine manufacturing facility network, which aims to expand pandemic response preparedness aligned with the 100 Days Mission and support equitable access to outbreak vaccines, particularly in Global South countries. This new funding is awarded as part of that initial agreement.
Additionally, the Houston Methodist Research Institute in Texas has combined its AI technology with established laboratory techniques to accelerate the development of broadly protective H5 vaccine candidate antigens.
Our Trust Standards: Medical Advisory Committee