$30 Million Makes the Zaire Ebolavirus Vaccine More Accessible
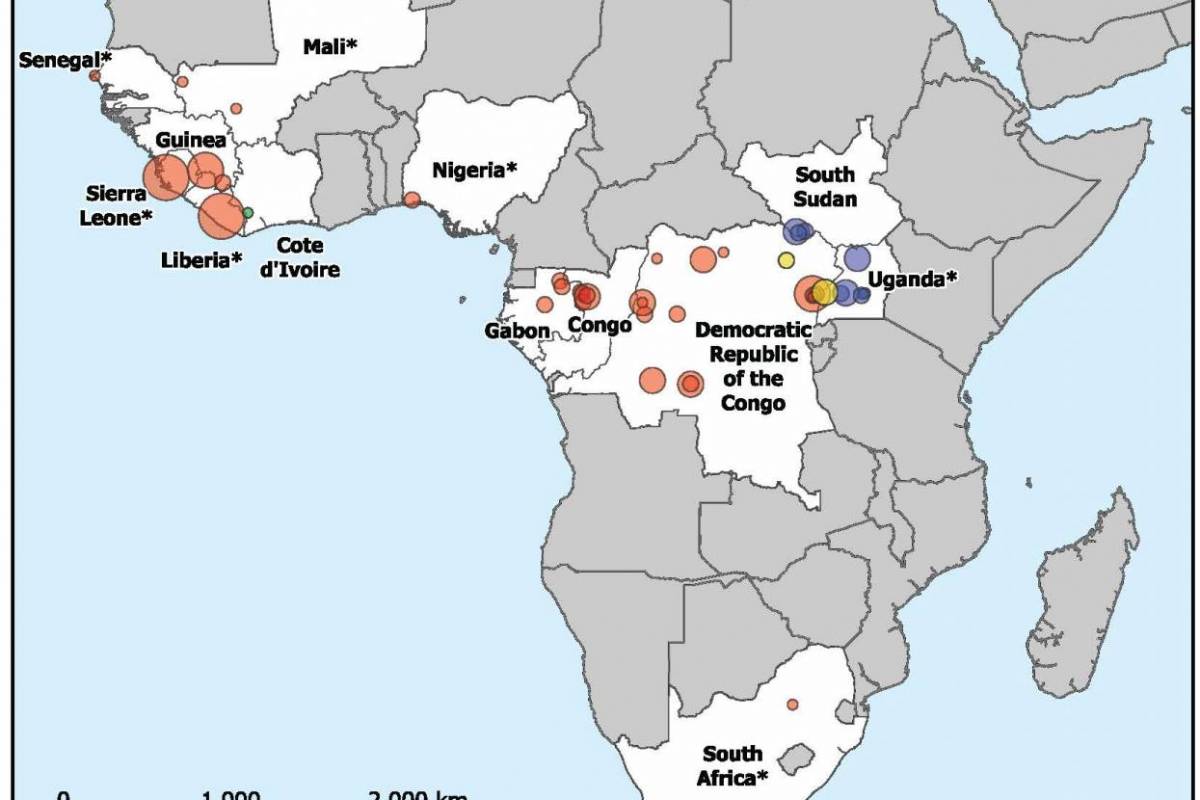
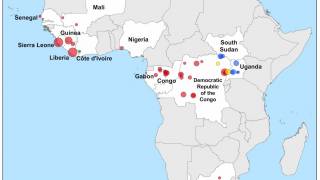
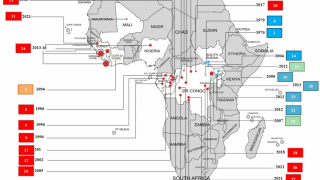
Ebola disease was first identified in 1976. Since then, outbreaks have emerged periodically and infected people in several African countries
A vaccine that has already received approval and is stockpiled for use against the Zaire ebolavirus could become more affordable and easier to deploy in low-resource settings due to a new collaboration between CEPI and MSD.
With the support of up to $30 million in funding from CEPI, MSD, also known as Merck in the United States and Canada, will utilize Hilleman Laboratories—a joint venture between MSD and Wellcome—to develop the Ervebo® Ebola vaccine with an updated manufacturing process.
Furthermore, SK bioscience and IDT Biologika will collaborate to develop the updated drug-substance process and the associated drug product.
"In a single decade the world has transformed Ebola from a global emergency to a disease that can be stopped in its tracks – and now CEPI's support will help to enable a sustainable and accessible supply of MSD's Zaire ebolavirus vaccine for years to come at a more affordable price," explains Dr. Richard Hatchett, CEO of CEPI, in a press release on January 21, 2026.
"This deal brings together longstanding partners of CEPI with longstanding partners of MSD to boost global defences against one of the deadliest pathogens known to humankind, helping to save lives."
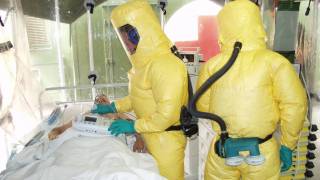
The existing MSD Zaire ebolavirus vaccine was developed in the midst of the 2014-16 West African Ebola crisis. Its manufacturing process is complex and potentially vulnerable to supply disruptions, making the vaccine expensive to produce and difficult to scale up. In addition, the vaccine must be stored in freezers at ultra-low temperatures of -70 degrees Celsius, creating substantial logistical challenges in the often remote, low-resource settings where Ebola outbreaks typically occur.
Currently, the Merck Ebola vaccine is not commercially available in the United States, but is maintained in the U.S. Strategic National Stockpile.
Our Trust Standards: Medical Advisory Committee