Bladder Cancer Therapy Demonstrates 96% Survival
For a few decades, the Bacillus Calmette-Guérin (BCG) vaccine has been a standard, effective, low-cost immunotherapy for non-muscle-invasive bladder cancer (NMIBC).
However, the 100-year-old BCG vaccine alone did not help all patients with bladder cancer recover.
Recently, the U.S. Food and Drug Administration approved ANKTIVA®, with BCG, for the treatment of patients with BCG-unresponsive NMIBC with carcinoma in situ (CIS), with or without papillary tumors.
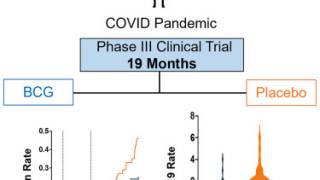
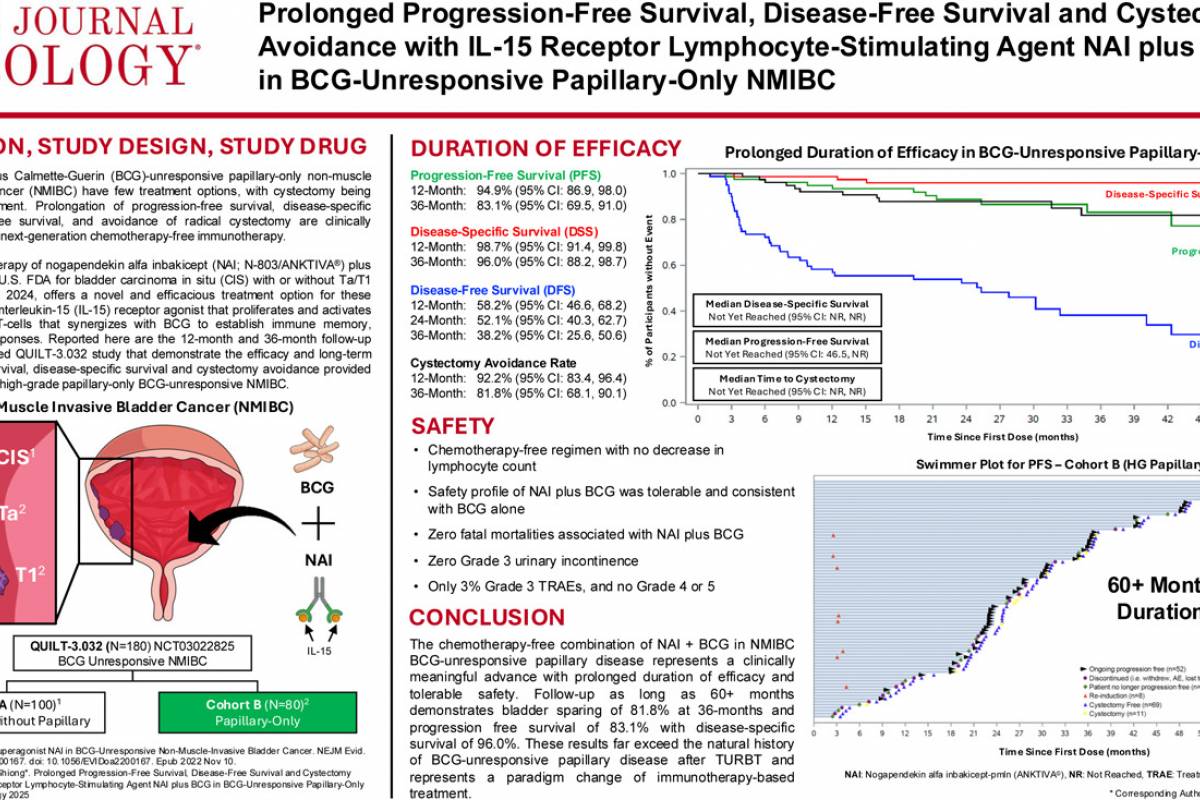
An Original Research article published in the Journal of Urology, January 1, 2026, edition, demonstrates efficacy at 12 and 36 months, including disease-free survival, disease-specific survival, long-term progression-free survival, and high cystectomy avoidance in patients with BCG-unresponsive high-grade papillary-only NMIBC.
These researchers stated the findings also show tolerable safety consistent with BCG treatment alone, with 3% grade 3 and no grade 4 or 5 treatment-related adverse events.
"Patients with BCG-unresponsive papillary-only non-muscle invasive bladder cancer have few treatment options, with cystectomy being considered the definitive treatment," said lead author Sam S. Chang, M.D., Professor of Urology and Chief Surgical Officer of the Vanderbilt Ingram Cancer Center, in a press release on December 16, 2025.
"Prolongation of progression-free survival, disease-specific free survival, and avoidance of bladder removal are clinically meaningful goals of next-generation chemotherapy-free immunotherapy."
"Our findings provide evidence that ANKTIVA plus BCG would offer a novel and efficacious treatment option for these patients."
ANKTIVA is currently approved in the USA and the United Kingdom, and has a Conditional Marketing Authorization in the European Union, with BCG, for the treatment of patients with BCG-unresponsive NMIBC with CIS, with or without papillary tumors.
"The evidence that CIS and papillary disease are clonally linked, combined with the QUILT-3.032 findings showing long-term cystectomy avoidance, sustained avoidance of progression to muscle-invasive disease, and 96% bladder cancer-specific survival at three years, supports the consideration that ANKTIVA plus BCG addresses the unmet need for patients with papillary disease alone who face the prospect of total radical cystectomy following failure of BCG therapy," added Dr. Patrick Soon-Shiong, Founder, Executive Chairman and Global Chief Scientific and Medical Officer of ImmunityBio.
Our Trust Standards: Medical Advisory Committee