Zika Vaccine Candidate Successfully Enhances Immune Response

The first human cases of the Zika virus (ZIKV) were detected in 1952; researchers have been working to develop a preventive vaccine.
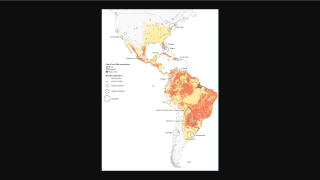
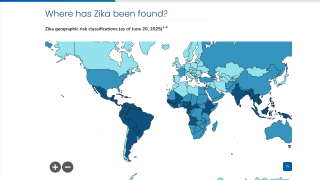
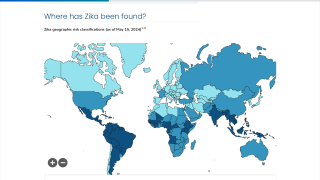
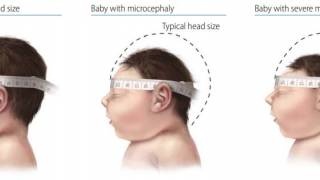
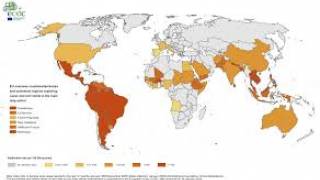
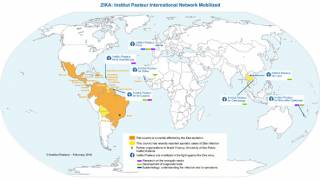
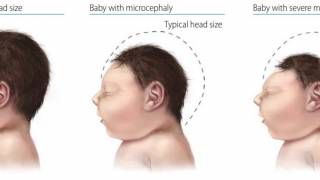
This effort is essential as 31 countries and territories have reported cases of congenital microcephaly and other central nervous system malformations associated with Zika virus infection.
To address the unmet medical need, Valneva SE, a France-based specialty vaccine company, has been conducting phase 1 clinical trials, including its second-generation candidate.
Today, Valneva announced positive results of its current Phase 1 clinical trial (VLA1601-102) investigating the safety and immunogenicity of VLA1601, its second-generation adjuvanted inactivated vaccine candidate against Zika.
As of November 4, 2025, the Company confirmed that two doses of VLA1601 were immunogenic across all five treatment arms investigated (i.e., alumadjuvanted Low, Medium, and High antigen dose; Low with additional adjuvants).
The strongest immune response was observed in the double-adjuvant treatment arms (Low+alum+3M-052-AF and Low+alum+CpG1018) with statistically significantly higher neutralizing antibody titers (Geometric Mean Titers - GMTs) at Day 43 and Day 57 than in the single-adjuvant (alum) treatment arm.
And the immune response induced by the double-adjuvanted VLA1601 was successfully improved compared to the first-generation vaccine candidate, with higher peak seroconversion rates (>93% vs 86%) and peak Geometric Mean Fold Increase in titers (> 56-fold vs > 7-fold).
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release, "We are pleased by the notable safety and immunogenicity results demonstrated for our Zika vaccine candidate and especially our double-adjuvantation results."
Should this vaccine development effort achieve US-FDA approval, international travelers, especially pregnant women, would be very interested in discussing immunization options with healthcare providers. Since 2013, 31 countries and territories, including Costa Rica and Puerto Rico, have reported cases of congenital microcephaly and other central nervous system malformations associated with Zika virus infection.
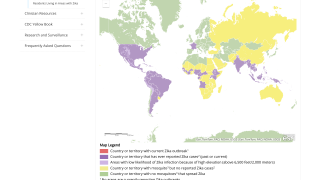
As of November 4, 2025, over 24,000 Zika cases and four related fatalities have been reported in the Region of the Americas this year.
Our Trust Standards: Medical Advisory Committee