Indonesia Testing Tuberculosis Vaccine Candidates
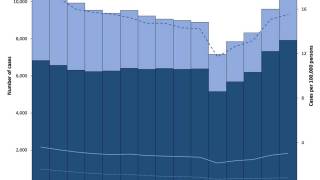
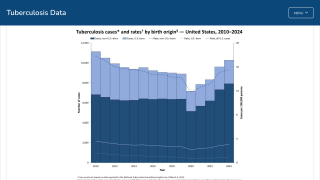
While the Republic of Indonesia has been battling tuberculosis cases for years, its focus in 2024 has turned to testing various vaccine candidates to curtail future outbreaks.
Despite having the second-highest number of tuberculosis cases, Indonesia has not participated in all previous vaccine studies.
According to an ANTARA News report on September 26, 2024, Indonesia is conducting clinical trials for three tuberculosis vaccines, including the M72/AS01E vaccine developed by the Bill & Melinda Gates Foundation.
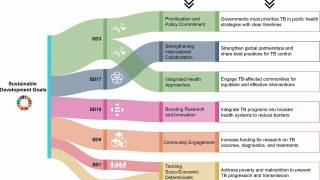
Indonesia's Health Minister Budi Gunadi Sadikin commented, 'the need for more discussions and conferences to eliminate tuberculosis by 2030, including by taking bold and aggressive action, especially in the vaccine development process.'
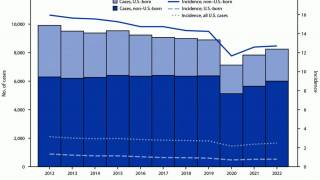
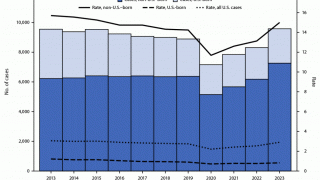
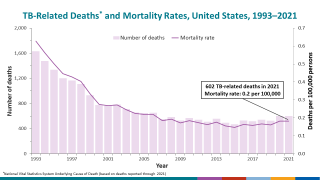
As of October 15, 2024, over ten tuberculosis vaccine candidates were being researched in various countries, including India, the unfortunate leader in TB cases.
In the United States, Merck's TICE BCG vaccine is FDA-approved for the prevention of tuberculosis; however, it has various access limitations, including those applicable when visiting Indonesia.
The U.S. CDC recommends pre-trip vaccinations for chikungunya, Japanese encephalitis, measles, and polio, but not TB, when visiting Indonesia in 2024. These travel vaccines are typically available at clinics and pharmacies in the United States.
Our Trust Standards: Medical Advisory Committee