Lyme Disease Vaccine Candidate Posts Positive Study Results

The Lancet Infectious Diseases recently published results from a phase 1 clinical trial of a Lyme disease vaccine candidate, showing that Valneva's VLA15 produces a strong but waning immune response against six common strains of the Borrelia burgdorferi bacterium found in Europe and the United States.
Valneva Austria researchers led this company-sponsored, partially randomized, observer-masked study of the novel, multivalent outer surface protein A (OspA) subunit vaccine candidate.
OspA is one of the most dominant surface proteins expressed by the bacteria when present in a tick.
VLA15 produced immune responses for all strains, but responses were greater in the higher-dose adjuvanted groups. And one month after the third dose, responses declined, reaching baseline by one year.
And a booster dose given 13 months after the first dose triggered a strong immune response for about six months.
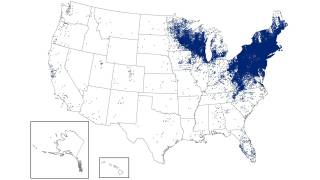
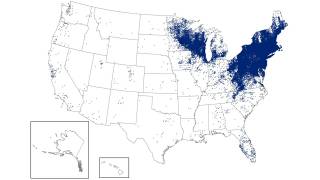
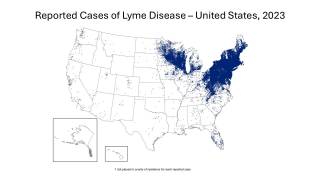
This study's findings are good news since Lyme borreliosis is the most common tick-borne disease in the northern hemisphere.
In Europe, there are estimated to be more than 200,000 cases each year. And in the U.S., approximately 30,000 patients per year.
In a related commentary also published by The Lancet, Nicole Bézay, and colleagues reported Valneva's novel vaccine represents a milestone in our fight against Lyme disease."
Initially developed by France-based Valneva SE, New York-based Pfizer, Inc. is VLA15's current development and commercialization collaboration.
Pfizer previously indicated it could submit a Biologics License Application in 2025 and Marketing Authorization Application in Europe in 2026, subject to positive data.
Our Trust Standards: Medical Advisory Committee