$18 Million Funds All-In-One Filovirus Vaccine
The Coalition for Epidemic Preparedness Innovations (CEPI) today announced that researchers will begin testing new vaccines that could provide all-in-one protection against viruses, including Zaire Ebolavirus, Sudan Ebolavirus, and Marburg virus, as well as future filoviruses.
A single vaccine capable of combating these threats could offer a cost-effective solution for proactively immunizing those most likely to be infected by one or more of the viruses.
On October 6, 2025, CEPI confirmed it will provide $18 million to fund new research conducted by scientists at the Stanford School of Medicine to study whether new vaccines could offer broad protection against the filovirus family.
"A broadly protective filovirus vaccine could transform the world's defences against outbreaks of some of the world's most dangerous pathogens, for the benefit of all," explains CEPI's Executive Director of Research & Development, Dr Kent Kester, in a press release.
"If we solve the scientific challenge of developing all-in-one filovirus vaccines now, we can ensure the world is ready to respond at speed to newly emerging filoviruses and potentially take the threat of future filovirus pandemics off the table."
The research will utilize artificial intelligence to design immunogens – the substance in a vaccine that provokes an immune response – that may be capable of protecting against multiple filoviruses.
These immunogens will be combined with a ferritin-based protein-nanoparticle vaccine platform to create a range of vaccine candidates that will be tested in preclinical studies to establish proof of concept.
The most promising vaccine candidate will be advanced to the point where it is ready to enter Phase I clinical trials should an unknown filovirus outbreak emerge.
The ferritin nanoparticle vaccine platform is favourable for use in low- and middle-income countries as it does not require complex frozen storage that can impact access to doses in low-resource settings. It has previously been tested in Phase I clinical trials for influenza and COVID-19 vaccines, which generated positive safety data.
Professor Peter S. Kim, the Virginia & D.K. Ludwig Professor of Biochemistry at the Stanford School of Medicine, and Principal Investigator on the project, commented, "We are excited to collaborate with CEPI to develop a globally effective broad-spectrum vaccine to protect against future filovirus pandemics."
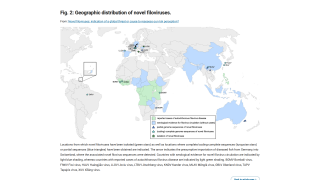
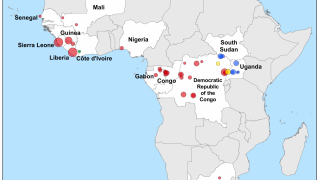
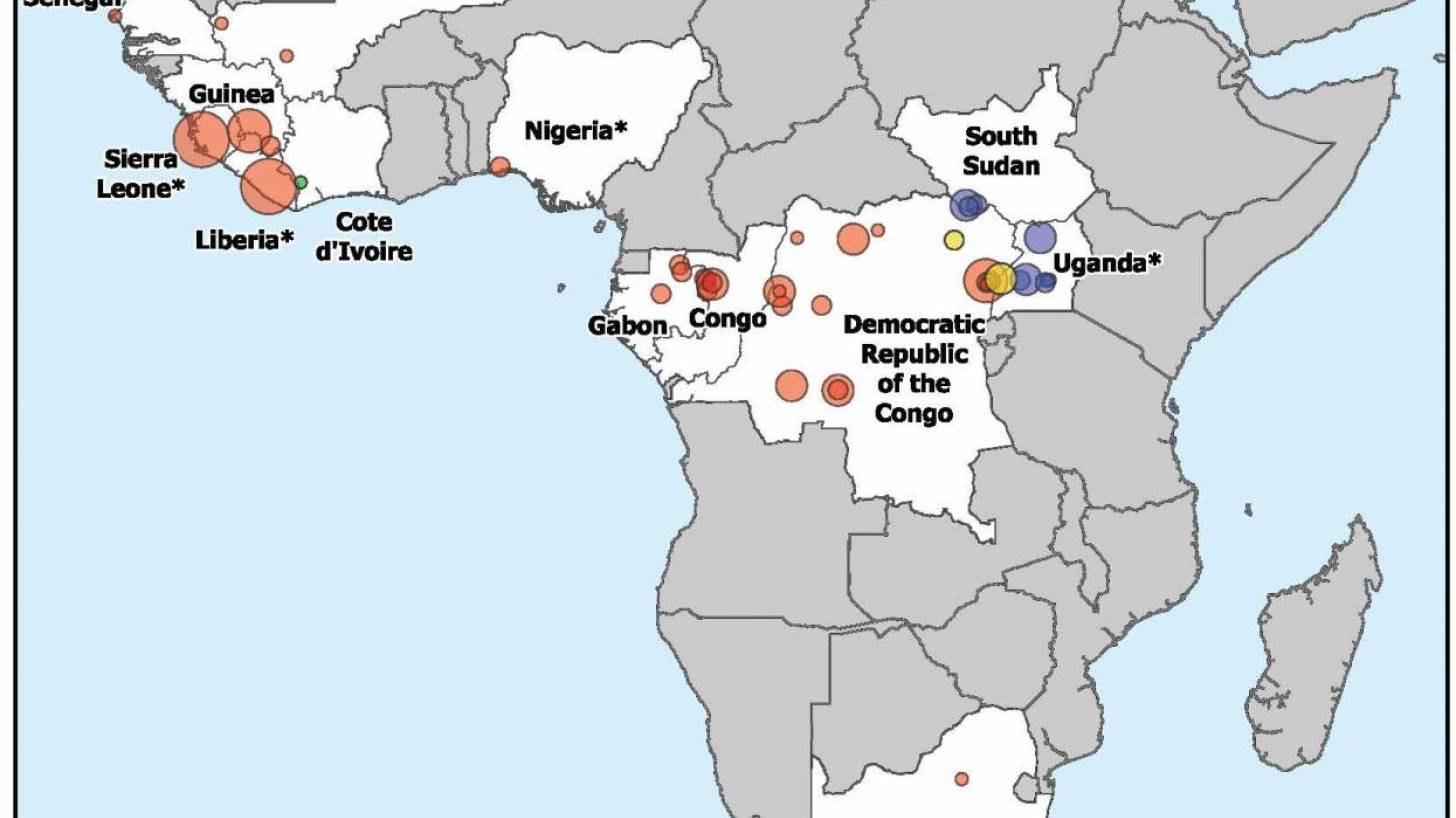
Filoviruses have primarily caused frequent outbreaks in African countries, characterized by high fatality rates and significant societal and economic impacts.
Ebola disease was first identified in 1976 in the Democratic Republic of Congo, which is currently confronting its 16th outbreak. Currently, vaccines and therapies have been approved for Ebola outbreaks in Africa.
The first Marburg outbreak was detected in Germany, and as of 2025, it does not have an approved vaccine. However, vaccine candidates are being tested in clinical studies.
Our Trust Standards: Medical Advisory Committee