Will 2026 Bring UTI Vaccines to the USA

As 2025 comes to a close, the number of men and women continuing to be impacted by urinary tract infections (UTIs) has ballooned by over 60% during the last decade.
This unfortunate trend disproportionately affects women.
In the United States, uncomplicated UTIs remain one of the most prevalent outpatient infections.
According to recent epidemiological data from the National Library of Medicine, about 50% of women experience at least one UTI by age 35. In comparison, up to 20% of younger women report at least one episode every year.
In the USA and the United Kingdom, UTI cases continue to reach new highs, even though vaccines have shown positive results in clinical trials.
Lauren E. Stewart, MD, a urogynecologist and reconstructive pelvic surgeon at NYU Langone Health, noted in an article published by IDSE on December 23, 2025, "There are vaccines being studied for preventing UTIs. To me, the clinical trials that are ongoing and are the most interesting at this time are focused on the development of vaccines for the prevention of UTIs."
"I think these studies show incredible promise and, if we can develop an effective vaccine, that could really improve a lot of lives," added Dr. Stewart.
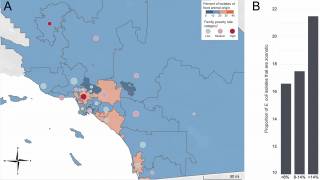
In the United Kingdom, the Health Security Agency (UKHSA) reports that UTI-related hospital admissions have surged, with 189,756 admissions with a primary diagnosis of UTI, a 9% increase from the previous reporting year.
This escalation has imposed a substantial financial strain on NHS hospitals, costing them an estimated £604 million during that period.
The UKHSA says these statistics underscore the need for UTI prevention measures, particularly vaccines targeting E. coli.
In the UK, vaccines such as Uromune™ (MV140), an oral spray vaccine, are commercially available at clinics near London. Other UTI vaccine options, such as Uro-vaxom and Solco-Urovac, are also available in the UK.
In the US, however, no UTI vaccines are approved as of December 27, 2025, though promising candidates are being tested in trials.
These studies include Sequoia Vaccines' rUTI vaccine candidate. And in August 2025, CARB-X awarded Baxiva AG $3 million to develop its multivalent glycoconjugate vaccine to prevent extraintestinal pathogenic Escherichia coli infections.
Looking ahead, government experts predict continued growth in UTI incidence unless preventive innovations such as vaccines gain broader regulatory approval. Currently, Uromune is offered in about 20 countries.
Researchers wrote in early 2025 that, as access to vaccines expands globally, UTI patients may soon benefit from reduced reliance on antibiotics, fostering a shift toward proactive immunity.
Our Trust Standards: Medical Advisory Committee