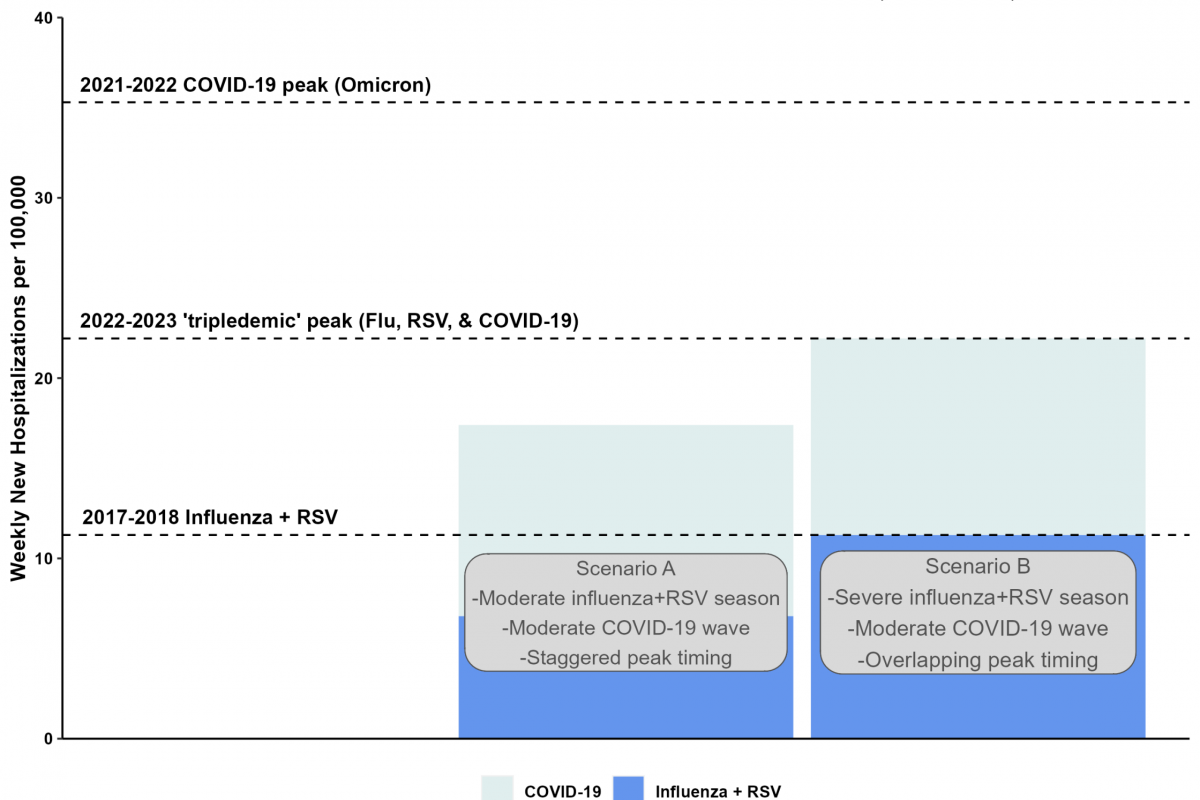
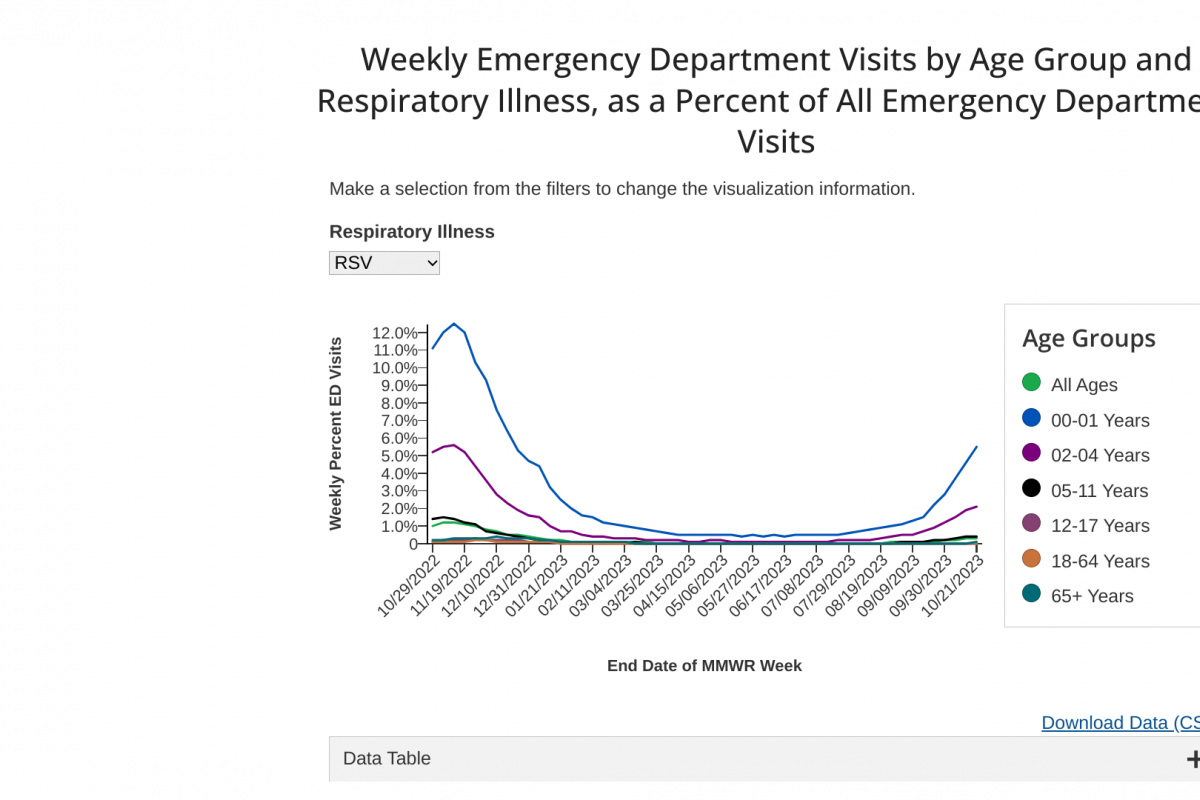
The Journal of Antimicrobial Chemotherapy recently published a study that suggests that influenza vaccinations are associated with less unnecessary antibiotic use in people over 65.
On October 28, 2023, these researchers concluded that seasonal flu shots might be a countermeasure against antimicrobial resistance (AMR) because they can reduce unnecessary antimicrobial use for acute respiratory infection by mitigating the burden of such diseases.
In total, 244,642 people were enrolled in this study in Tokyo, Japan.
The average treatment effect of vaccination was:
−0.004 (95% CI −0.006 to −0.002) for the frequency of antibiotic prescription,
−0.005 (−0.007 to −0.004) for the frequency of healthcare facility consultation,
−0.001 (−0.002 to −0.001) for the risk of admission and
- 0.00 (0.00 to 0.00) for the risk of death.
Our results suggest that the seasonal influenza vaccine might have indirect benefits for not only preventing influenza-like illnesses but also as a countermeasure against AMR, wrote these researchers.
However, because we included only an older population, we cannot know whether a similar effect would be seen if children or young adults were the target population for the vaccine.
Globally, AMR is one of the leading causes of death.
The World Health Organization says that AMR happens when microorganisms, such as bacteria, fungi, viruses, and parasites, change when exposed to antimicrobial drugs, such as antibiotics, antifungals, antivirals, antimalarials, and anthelmintics.
On October 19, 2023, the WHO released 13 interventions to guide country prioritization when developing, implementing, and monitoring national action plans on AMR.
These interventions address the needs and barriers people and patients face when accessing health services through a people-centered approach to AMR.