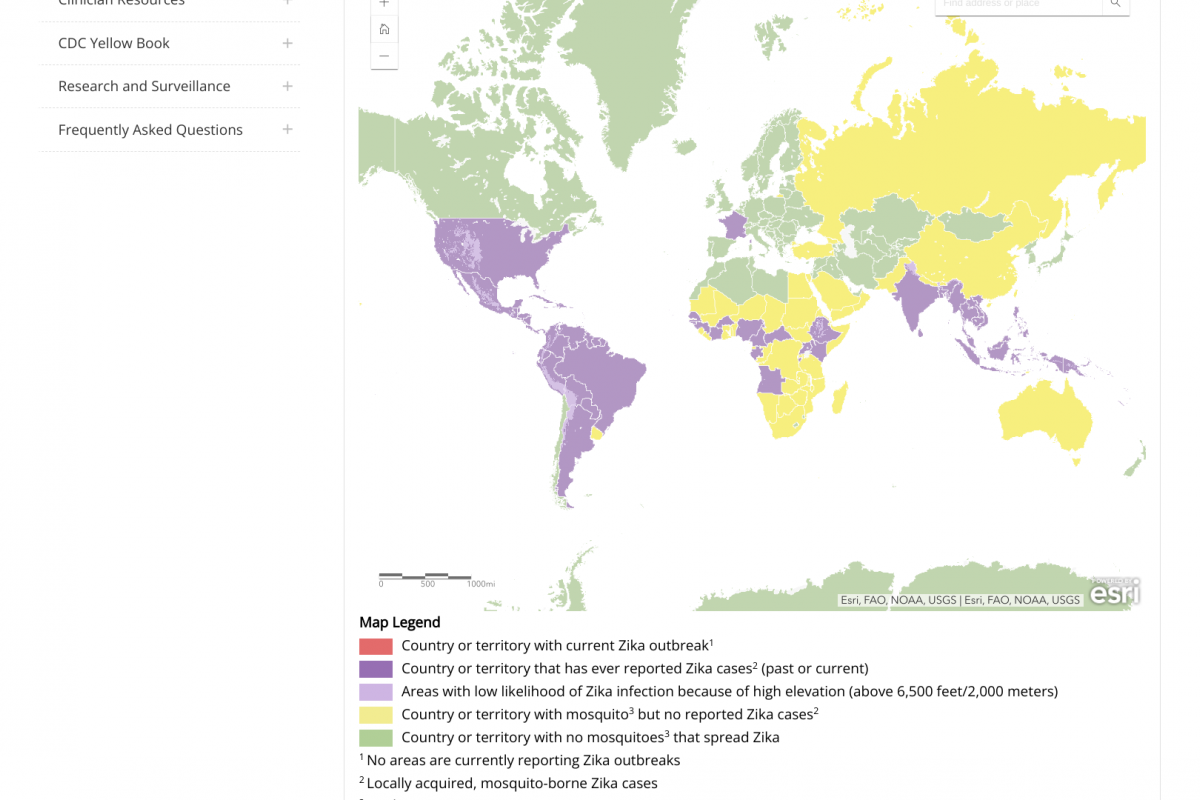
According to a recent study, a seasonal analysis revealed that Brazil's most significant risk of Zika and Chikungunya disease occurs during the summer when higher temperatures occur.
The resurgence was identified in northeast Brazil between 2019 and 2021 for Zika and in 2021 for Chikungunya.
As of November 8, 2023, over 26,659 Zika cases have been reported in Brazil this year.
Published by the journal Scientific Reports on October 21, 2023, this trend is related to the increased Ae. aegypti mosquito infestation levels due to the decreased time for larval development.
And the increased proportion of infectious mosquitoes, given the decreased intrinsic incubation periods of the viruses in the vector.
Studies conducted in China, the United States, and the Brazilian states of Rio de Janeiro and São Paulo also indicated that temperature influenced the distribution patterns of Ae. aegypti and Ae. albopictus, consequently affecting the incidence of diseases they transmit.
The small but significant differences (from 0.7 to 2.6 ∘C) in the average temperature between the high-risk and no-risk areas for both diseases are worth consideration, wrote these researchers.
For example, Banu et al. showed that an increase of 1 ∘C could be related to a future rise in arbovirus disease cases.
In recent decades, consistent and widespread warming has been observed throughout Brazil, with greater extreme heat occurring during spring and summer.
From a prevention perspective, Zika vaccine candidates continue in clinical trials. However, the U.S. FDA recently approved the first Chikunynga vaccine.