Vaccine-Enhancing Adjuvant Library Launches
Scientists have launched the first-ever library of adjuvants that can be 'taken off the shelf' and used to enhance new vaccines being developed against epidemic and pandemic threats.
Announced on July 31, 2025, the UK's Medicines and Healthcare products Regulatory Agency (MHRA) will host a repository of 25 vaccine-enhancing adjuvants. The adjuvant library could help quickly identify the top-performing vaccine-adjuvant pairings.
The MHRA is responsible for regulating all medicines and medical devices in the UK.
The $2.5 million project will act as a matchmaking service, helping vaccine developers select the best vaccine-adjuvant combinations to make their vaccines more potent and effective to contain the spread of a virus before it reaches pandemic proportions.
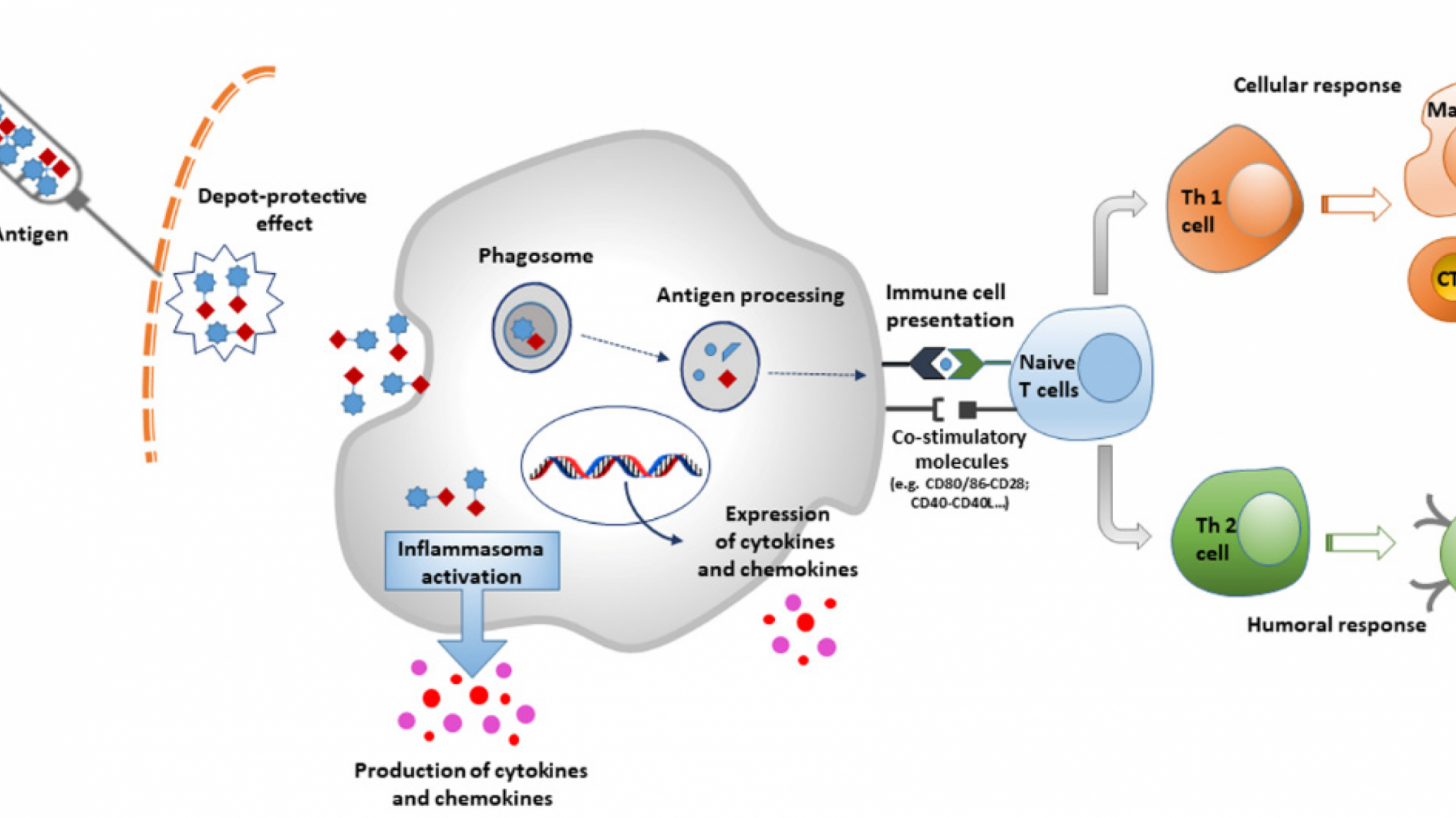
Named after the Latin word "adjuvare", vaccine-enhancing adjuvants have played a powerful role in transforming our response to deadly diseases over the past century. The ingredients are added to the majority of vaccines to enhance the immune response, creating stronger and longer-lasting protection against infections than the vaccine alone.
This service is funded and led by the Coalition for Epidemic Preparedness Innovations (CEPI).
Dr Richard Hatchett, CEO of CEPI, explained in a press release, "Constrained supplies can result in an adjuvant getting paired with a vaccine based on what's available at the time rather than what works best."
"This world-first library will fill the gap by matching vaccines to a range of adjuvants to more rapidly identify the best combinations that could save lives and even stop a future pandemic in its tracks."
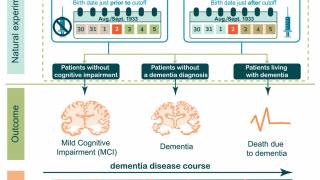
An example of how vaccines can enhance vaccines was presented in a Brief Communication published by NPJ Vaccines on June 25, 2025. This analysis reported a lower risk of dementia associated with AS01-adjuvanted vaccination against shingles.
Adjuvant developers applied to join the library following an Expression of Interest published by CEPI in 2023. In line with CEPI's Equitable Access Policy, vaccine developers are encouraged to publish data on their vaccine and adjuvant pairings in open-access publications for all to benefit from the research.
Our Trust Standards: Medical Advisory Committee