Shigellosis Vaccine Needed in Africa
A seldom discussed gastrointestinal infection caused by Shigella bacteria continues to pose significant public health challenges in Africa, Europe, and the United States.
Researchers recently reported in BMC Infectious Diseases that 29 African countries have a high burden of Shigellosis outbreaks and high levels of antibiotic resistance.
This Systematic Review, published on October 29, 2024, by researchers at the University of Ghana Medical School, included 116 studies examining 99,510 patient samples. The most prevalent species of Shigella was Shigella flexneri (53.6%).
The meta-analysis indicated that the overall pooled prevalence of Shigella bacteria across Africa was 5.9%, with regional prevalences of 6.9% in southern Africa, 6.7% in northern Africa, 6.2% in eastern Africa, 4.5% in central Africa, and 4% in western Africa.
Furthermore, Shigella prevalence was higher in children (6.6%) than in adults (3.6%).
And the resistance to ampicillin, trimethoprim-sulfamethoxazole, and chloramphenicol was high (77.8%, 65.1%, and 45.2%, respectively), while resistance to ceftriaxone (8.5%) and ciprofloxacin (10%) was low.
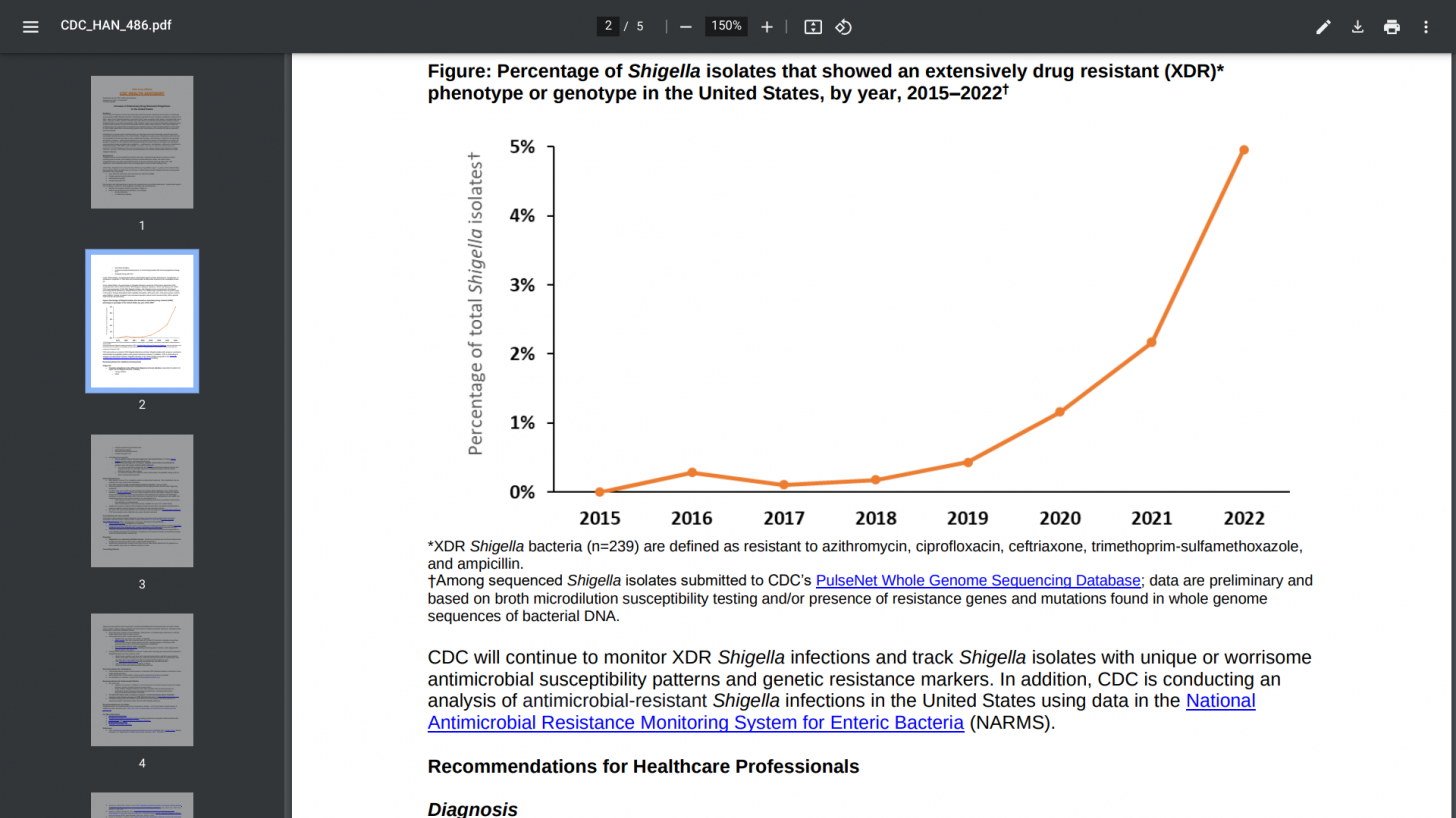
In the United States, The Centers for Disease Control and Prevention (CDC) has been monitoring an increase in extensively drug-resistant (XDR) Shigella infections (Shigellosis). The CDC confirmed in 2024 that the Shigella bacteria can spread quickly. People with Shigella infection can shed the bacteria in their stool for weeks after symptoms have dissipated.
As of October 26, 2024, the CDC confirmed 17,709 Shigella cases, led by California (L.A.) and New York (NYC). In 2023, the CDC reported 16,602 cases in the U.S.
Additionally, the European CDC reported 4,149 confirmed cases of Shigellosis in 2022. France, the Netherlands, and Spain accounted for 50.6% of these cases
These researchers wrote, 'Vaccine development is a prioritized area; however, progress has been impeded by the diverse range of Shigella serotypes and the complexity involved in developing effective vaccines.'
To address this public health need, the U.S. FDA recently Fast-Tracked an innovative Shigellosis vaccine candidate.
Valneva SE and LimmaTech Biologics AG are co-developing Shigella4V (S4V), a tetravalent bioconjugate vaccine candidate against Shigellosis. The anticipated FDA regulatory pathway for S4V is leveraging a combination of Controlled Human Infection Model studies to support potential initial approval in adults, followed by field efficacy studies to expand the indication to children.
As of November 4, 2024, the future availability of the S4V vaccine is undisclosed.
Our Trust Standards: Medical Advisory Committee




