Shigella Vaccine Candidate Awarded U.S. FDA Fast Track Designation

A seldom-discussed global health threat may soon have a U.S. Food and Drug Administration (FDA)- approved vaccine available.
Valneva SE and LimmaTech Biologics AG today announced that the U.S. FDA has granted Fast Track designation to Shigella4V (S4V), the world's most clinically advanced tetravalent bioconjugate shigellosis vaccine candidate.
Fast Track designation facilitates clinical development and expedites the review of essential new products, intending to get them to those who need them earlier, such as people diagnosed with Shigellosis.
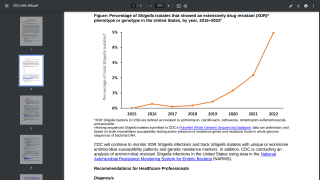
Shigellosis may cause up to 165 million infections and 600,000 deaths each year. The Gram-negative Shigella bacteria is the second leading cause of diarrheal deaths.
Shigellosis affects international travelers from high-income countries, deployed military personnel in endemic regions, and children in Low- and Middle-Income Countries.
Thomas Lingelbach, CEO of Valneva, commented in a press release on October 16, 2024, "Shigellosis is the second leading cause of fatal diarrheal disease worldwide. However, there is currently no approved Shigella vaccine, and the development of Shigella vaccines has been identified as a priority by the World Health Organization (WHO)."
"Fast Track designation allows us to work closely with the FDA to accelerate our efforts to deliver a preventative solution against this deadly disease."
In August 2024, Valneva entered into a strategic partnership and exclusive licensing agreement with LimmaTech to develop, manufacture, and commercialize S4V. Following positive Phase 1/2 results earlier in 2024, LimmaTech will conduct a Phase 2 Controlled Human Infection Model study (CHIM) in the U.S. and a Phase 2 pediatric study in LMICs, both expected to begin before the end of 2024.
Once approved, Valneva will assume responsibility for all further development, including chemistry, manufacturing, controls, and regulatory activities, and will be responsible for worldwide commercialization.
The anticipated FDA regulatory pathway for S4V will leverage a combination of CHIM studies to support potential initial approval in adults, followed by field efficacy studies to expand the indication for children.
Until a vaccine becomes available, the WHO says the standard treatment for Shigellosis is oral rehydration and antibiotic therapy.
However, the bacteria have acquired resistance to many antibiotics, and numerous reports of outbreaks of multidrug-resistant strains have made treatment extremely difficult.
Valneva SE is a specialty vaccine company that develops, manufactures, and commercializes prophylactic vaccines for infectious diseases, addressing unmet medical needs, such as chikungunya and Lyme disease.
Our Trust Standards: Medical Advisory Committee