Shigella Vaccine Candidate Advancing in Clinical Trials

Shigella, the leading cause of diarrheal deaths worldwide, is having a resurgence in 2024. Among young children, it is estimated that diarrhea caused by Shigella is responsible for about 446,000 deaths globally each year.
In the United States, the Centers for Disease Control and Prevention (CDC) reported 16,498 Shigella cases on October 5, 2024 (Week 40), indicating an 8% increase from 2023.
California (3,483), New York (2,478), and New Jersey (1,446) are leading other states.
Recent news from Illinois, California restaurants, and Florida Zoo primates are being infected with Shigella this year.
Currently, Shigella lacks a U.S. FDA-approved licensed vaccine.
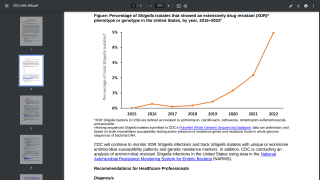
World Health Organization (WHO) says Shigella are bacteria that cause people to get sick when eating or drinking contaminated food or water. Since treatment options for shigellosis include antimicrobials, the rise of antimicrobial-resistant enteric bacteria, particularly Shigella, means that, in addition to the potential direct effects on morbidity and mortality, a Shigella vaccine might also have indirect effects on reducing the use of antibiotics and the consequent emergence of antimicrobial resistance, a global concern in 2024.
As of October 2024, several European countries have reported an emerging multidrug-resistant Shigella sonnei ST152 strain. Additionally, the UK has reported 268 cases belonging to the same strain.
Therefore, the WHO says developing a Shigella vaccine is an essential goal for public health.
To meet this challenge, Juan Carlos Jaramillo, M.D., the Chief Medical Officer of Valneva SE, commented on October 10, 2024, that the Company 'In-licensed' Limmatech's S4V tetravalent bioconjugate Shigella vaccine candidate and would soon focus on a Phase 3 clinical trial program.
LimmaTech previously reported positive Phase 1/2 results for S4V, including a favorable safety and tolerability profile and robust data on immunogenicity against the four most common pathogenic Shigella serotypes: S. flexneri 2a, 3a, 6, and S. sonnei.
While Valneva did not forecast an availability date, late-stage clinical studies generally last over one year to compile the required data to obtain FDA approval.
Our Trust Standards: Medical Advisory Committee