Search API
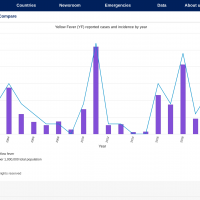
Dynavax Technologies Corporation today confirmed it continues developing a plague (rF1V) vaccine candidate adjuvanted with CpG 1018® in collaboration with, and fully funded by, the U.S. Department of Defense (DoD).
As of February 20, 2025, based on the results from a randomized, active-controlled Phase 2 clinical trial, Dynavax and the DoD executed a new agreement for approximately $30 million through the first half of 2027 to support additional clinical and manufacturing activities, including a Phase 2 clinical trial expected to initiate in the third quarter of 2025.
As previously announced, Dynavax and the DOD executed an earlier agreement providing approximately $22 million in funding to develop the rF1V vaccine.
According to the U.S. CDC, Plague is a potentially deadly infectious disease caused by bacteria found in fleas and rodents or by handling an infected animal. It is caused by the bacterium Yersinia pestis. It is possible that Pneumonic plague bacteria could be released intentionally in a biological attack to sicken people.
Since the mid–20th century, plagues in the United States have typically occurred in the rural West. The CDC says cases in the eastern United States are among people who traveled from the west or have laboratory exposure.
More recent plague epidemics have occurred in Africa, Asia, and South America.
Throughout the record-setting Dengue fever outbreak in the Region of the Americas in 2024, most international travelers were unprotected when visiting at-risk countries such as Brazil, Costa Rica, and even Miami Breach.
Furthermore, once infected with this mosquito-transmitted viral disease, patients could not access innovative therapies.
To accelerate the approval of Dengue treatments, the U.S. National Institutes of Health (NIH) is testing an experimental treatment.
The phase 2 clinical study will involve exposing volunteers to a weakened strain of dengue virus that causes a mild form of the disease and administering an investigational therapeutic at various doses to assess its safety and ability to lessen symptoms.
This NIH clinical trial will test the ability of AV-1, an investigational human monoclonal antibody (mAb) therapeutic developed by AbViro, to mitigate clinical symptoms when administered before and after dengue virus infection at Johns Hopkins Bloomberg School of Public Health Center for Immunization Research and the University of Vermont.
However, none of the volunteers will develop dengue fever or severe dengue during this study.
“When caring for a patient who is critically ill with dengue, healthcare providers have few options other than providing supportive care,” said NIAID Director Jeanne Marrazzo, M.D., M.P.H., in a press release on February 11, 2025.
“We must find safe and effective therapeutics to provide much-needed relief to people suffering from dengue.”
The researchers will use this information to determine how AV-1 affects the volunteers’ ability to recover from dengue compared to placebo and to determine the dosages at which AV-1 may be effective.
If AV-1 shows promising results in this clinical trial, researchers may pursue further clinical evaluations of its safety and efficacy against the dengue virus.
The results of a previously completed Phase 1 trial indicated that AV-1 is safe in humans, providing the basis for the new clinical trial to test its safety and efficacy.
Another Dengue mAb candidate, Dengushield (VIS513), is being evaluated in phase 2 clinical trials.
This mAb is a highly potent inhibitor of all four types of dengue viruses, both in vitro and in preclinical animal models. Dengushield was licensed to the Serum Institute of India Pvt. Ltd. for development.
Despite these potential innovations, during Spring Break 2025, preventing mosquito bites is the best tactic to avoid Dengue infection.

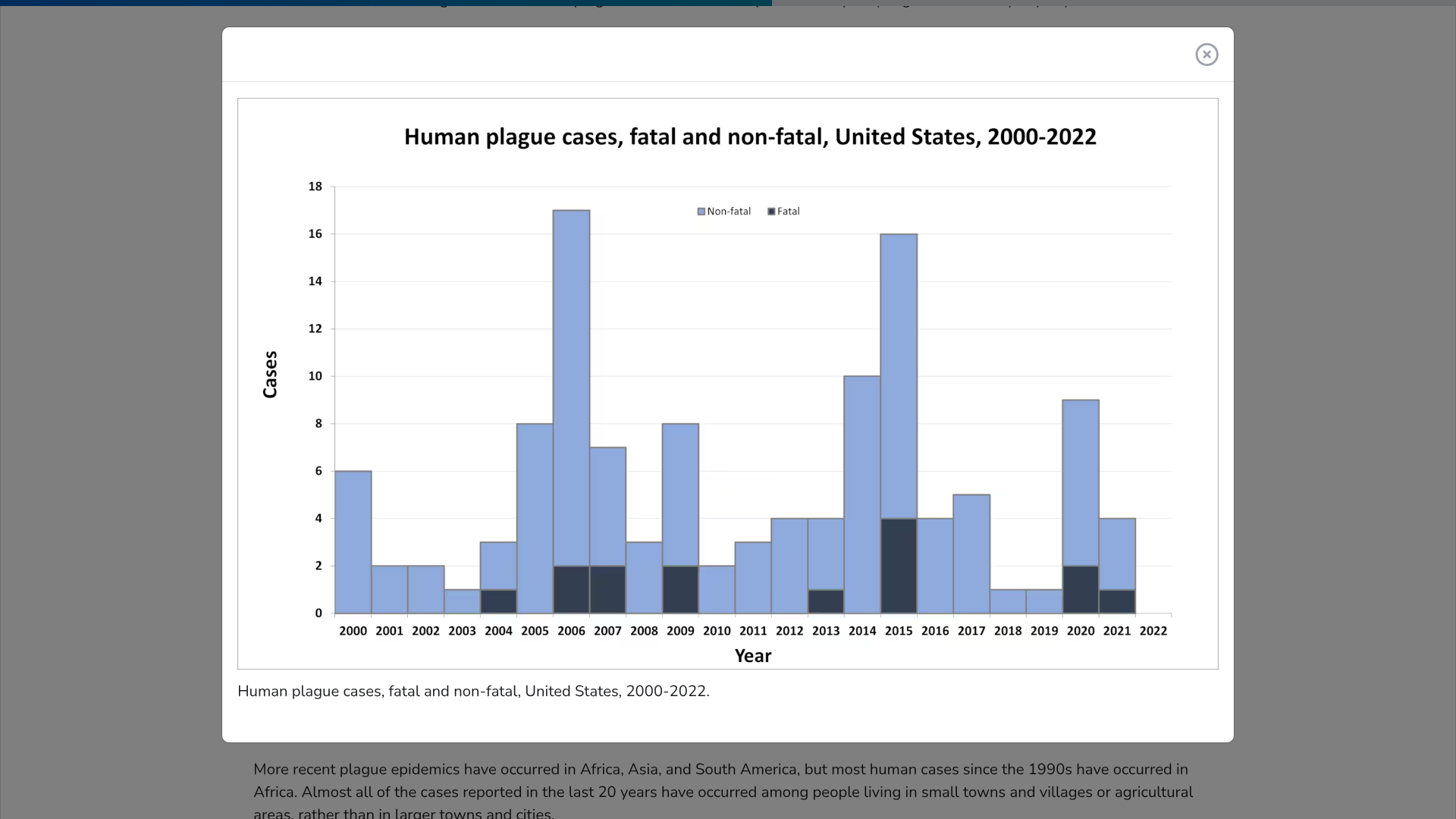
The World Health Organization (WHO) today announced two very positive trends related to the 7th cholera global outbreak.
On February 20, 2025, the WHO confirmed a 27% decrease in cholera cases since December 2024. A total of 34,799 new cholera and/or Acute Watery Diarrhoea cases were reported from 19 countries, territories, and areas across three WHO regions.
The period also saw a 33% decrease in related fatalities from the previous month.
This decrease was recorded despite a new cholera outbreak in Angola.
Additionally, the WHO reported that in January 2025, Oral Cholera Vaccine (OCV) production reached 6.2 million doses, a recent high point compared to December 2024, when 5.5 million doses were produced.
This progress follows introducing and prequalifying a new vaccine formulation and manufacturing process earlier in 2024.
However, the current OCV production has yet to meet growing global demand, and demand continues to exceed supply, says the WHO.
In the United States, OCVs will be offered at travel clinics and pharmacies in 2025.
The U.S. CDC's Advisory Committee on Immunization Practices (ACIP) today announced it has postponed next week's vaccine review meeting. This ACIP meeting was scheduled from February 26 to 28, 2025.
The draft agenda had been posted in early February.
As of February 20, 2025, the CDC's website stated, 'The ACIP workgroups met as scheduled this month and will present at the upcoming ACIP meeting.'
The next regularly scheduled ACIP meeting is in late June 2025.
The ACIP holds about three annual meetings, which are open to the public. At these meetings, scientists review scientific data and vote on vaccine recommendations. The ACIP recommendations are then passed on to the CDC's Director, who makes the final decisions.

The World Health Organization says streptococcus pneumoniae is a leading cause of vaccine-preventable deaths globally, and new, innovative vaccines are needed to curtail this disease.
To address this need, Vaxcyte, Inc. recently announced that the first study participants had been dosed in the second and final stage of the ongoing Phase 2 study of VAX-31 in healthy infants.
This clinical study evaluates the safety, tolerability, and immunogenicity of VAX-31, a 31-valent pneumococcal conjugate vaccine (PCV) candidate designed to prevent invasive pneumococcal disease (IPD) in healthy infants.
“PCVs have demonstrated the ability to deliver herd immunity to protect against devastating diseases caused by Streptococcus pneumoniae bacteria, and our technology has the potential to deliver best-in-class PCVs with broader coverage for both infants and adults,” said Jim Wassil, Executive Vice President, and Chief Operating Officer of Vaxcyte, in a press release on February 5, 2025.
“Based on the body of positive evidence from the VAX-31 and VAX-24 adult Phase 1/2 programs, we believe our carrier-sparing platform has the potential to set a new standard in disease coverage.”
The Company expects to share topline data from the primary three-dose immunization series of the study in mid-2026, followed by topline data from the booster dose approximately nine months later.
In children under five. Pneumococci also cause over 50% of all cases of bacterial meningitis in the U.S. And pneumococcal pneumonia is estimated to result in approximately 150,000 hospitalizations yearly.
And Streptococcus pneumoniae is among the top antibiotic-resistant pathogens to be urgently addressed, and the U.S. CDC lists drug-resistant Streptococcus pneumoniae as a “serious threat.”
This indicates the need for expanding PCV vaccinations is essential.

With the detections of the Eurasian H5 strain of highly pathogenic avian influenza (HPAI) in wild birds, domestic poultry, and mammals over the past few years, Canada took action today to prepare for a pandemic.
As of February 19, 2025, the Public Health Agency of Canada (PHAC) and its partners have secured an initial supply of 500,000 doses of GSK's human vaccine against HAPI infections – Arepanrix H5N1 A/American wigeon clade 2.3.4.4b.
This U.S. FDA-approved vaccine will be part of Canada's contingency planning. PHAC will provide vaccines to provinces and territories using an equitable and risk-based approach. Sixty percent of available doses will go to provinces and territories, and 40 percent will be kept in a federal stockpile.
It is common to detect avian influenza in wild birds, as viruses circulate freely in those populations without the birds appearing sick. While the current risk to the public remains low, individuals with higher-level exposure to infected animals are at increased risk for avian influenza.
Dr. Theresa Tam, Canada's Chief Public Health Officer, commented in a press release, "By making human vaccines against avian influenza available for potential use in individuals at increased risk of exposure to avian influenza as part of our readiness, we are enhancing our capacity to protect people in Canada and respond rapidly to emerging public health challenges."
The health agencies of the United States, the United Kingdom, Japan, Europe, and China have already approved and purchased avian influenza vaccines.

The continued detection of poliovirus in wastewater in 2025 signals the ongoing risk to children in the Gaza Strip. The virus poses a severe risk to children with low or no immunity throughout the region.
As poliovirus is found to remain in the environment, additional vaccination efforts are needed to strengthen population immunity.
Today, the World Health Organization (WHO) announced a third polio vaccination campaign to protect more children from this debilitating disease.
As of February 19, 2025, the WHO confirmed that over 591,000 children under 10 will receive the novel oral polio vaccine type 2 (nOPV2) in late February to protect them from polio.
Two previous vaccination rounds in the Gaza Strip were conducted in September and October 2024, and they reached over 95% of the target.
According to the WHO and the Global Polio Eradication Initiative, the nOPV2 vaccine, which has been 'triple-locked' using genetic engineering to prevent it from becoming harmful and producing a mutation, has been deployed over 1 billion times over the last few years.
To alert international travelers to this polio risk, the U.S. CDC updated its Level 2 Practice Enhanced Precautions, Global Polio Advisory, on January 14, 2025. The CDC says that before any trip to known poliovirus areas, you should ensure you are up to date on your polio vaccines.
In the United States, the single-antigen inactivated poliovirus vaccine has been available at clinics and pharmacies since 2000.