Search API
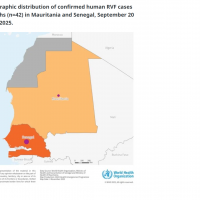
The Government of the Hong Kong SAR's Center for Health Protection (CHP) of the Department of Health today announced a third locally acquired case of chikungunya fever (CF) in a middle-aged man living in the Kwai Tsing District and working in Sheung Wan.
As of November 4, 2025, the CHP will conduct genome analysis to determine whether it has epidemiological linkage to the imported cases confirmed earlier in Hong Kong. Investigation is ongoing.
Kong Kong's initial CF case was reported on October 28, 2025.
In response to the new local case, the CHP today has conducted an inter-departmental meeting with various departments and relevant organisations.
To alert international travelers to the expanding CF health risk in this region, the U.S. CDC published a Level 2—Practice Enhanced Precautions —Travel Health Notice in late August 2025.
The CDC stated that you can protect yourself by preventing mosquito bites, which includes using insect repellent, wearing long-sleeved shirts and pants, and staying in places with air conditioning or with screens on the windows and doors.
Additionally, the CDC recommended vaccination for certain travelers visiting areas with chikungunya outbreaks. In general, vaccination against chikungunya should be deferred until after delivery for pregnant women.
In the United States, approved chikungunya vaccines are commercially available at travel clinics and pharmacies.
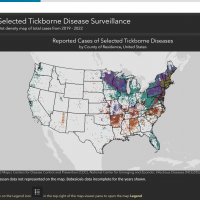
The Texas Department of State Health Services (DSHS) recently announced a significant increase in pertussis (whooping cough) cases.
According to provisional DSHS data updated on November 3, 2025, Texas has had more than 3,500 reported pertussis cases through October this year, roughly four times the number (1,907) reported for the same period last year, which realized a spike in November and December.
Texas reported 340 cases in 2023.
This is the second consecutive year Texas has experienced high year-over-year increases in reported pertussis cases, and the second successive year DSHS has issued a health alert.
DSHS wrote that pertussis can cause serious and potentially life-threatening complications in infants and young children who are not fully immunized.
The best way to prevent pertussis is to get vaccinated. However, immunized children and adults can still get pertussis, so a history of immunization does not rule out a pertussis diagnosis. Immunized children, adolescents, and adults may present with milder symptoms and lack the classic "whoop", says DSHS.
Across the United States, preliminary CDC data for 2024 show that more than six times as many cases were reported as in 2023.
In Texas, pertussis vaccines are offered at health clinics and pharmacies.

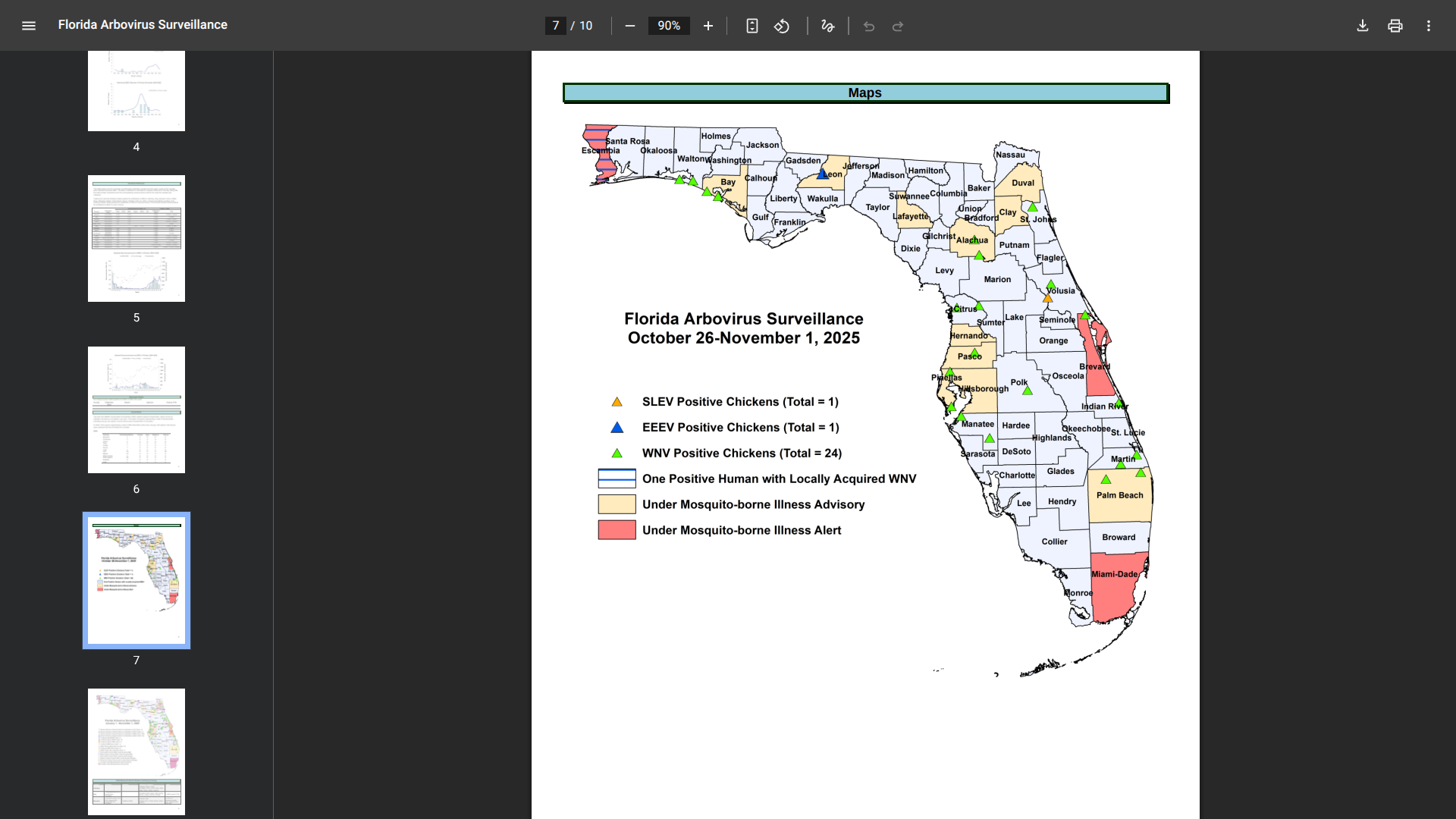
The Florida Department of Health recently published its Vaccine-Preventable Diseases Surveillance Report #44 for 2025, revealing a continuation of locally acquired and travel-related mosquito-transmitted disease cases.
As of November 1, 2025, Chikungunya, Dengue, and Malaria patients were primarily identified along Florida's southeast coast.
This year, 42 travel-related Chikungunya cases have been confirmed in Broward, Collier, Lake, Miami-Dade, Palm Beach, and Seminole counties. The countries of origin included Bolivia, Brazil, Cuba, India, and Indonesia.
Also, the health department reported 321 cases of Dengue fever among individuals who had traveled internationally, with over 180 cases related to travelers from Cuba and 14 from Puerto Rico. Additionally, 53 locally acquired Dengue cases were reported in Hillsborough, Miami-Dade (16), Pasco, and Brevard (35) counties.
Furthermore, 41 travel-related Malaria cases have been confirmed in Florida, 15 related to travel to Nigeria.
To alert visitors to these health risks, Alachua, Bay, Clay, Duval, Hillsborough, Lafayette, Leon, Pasco, and Pinellas counties are under a mosquito-borne illness advisory. Brevard, Escambia, and Miami-Dade Counties remain under a mosquito-borne illness alert.
However, the U.S. CDC has not issued a Travel Health Notice for Florida regarding these disease risks.
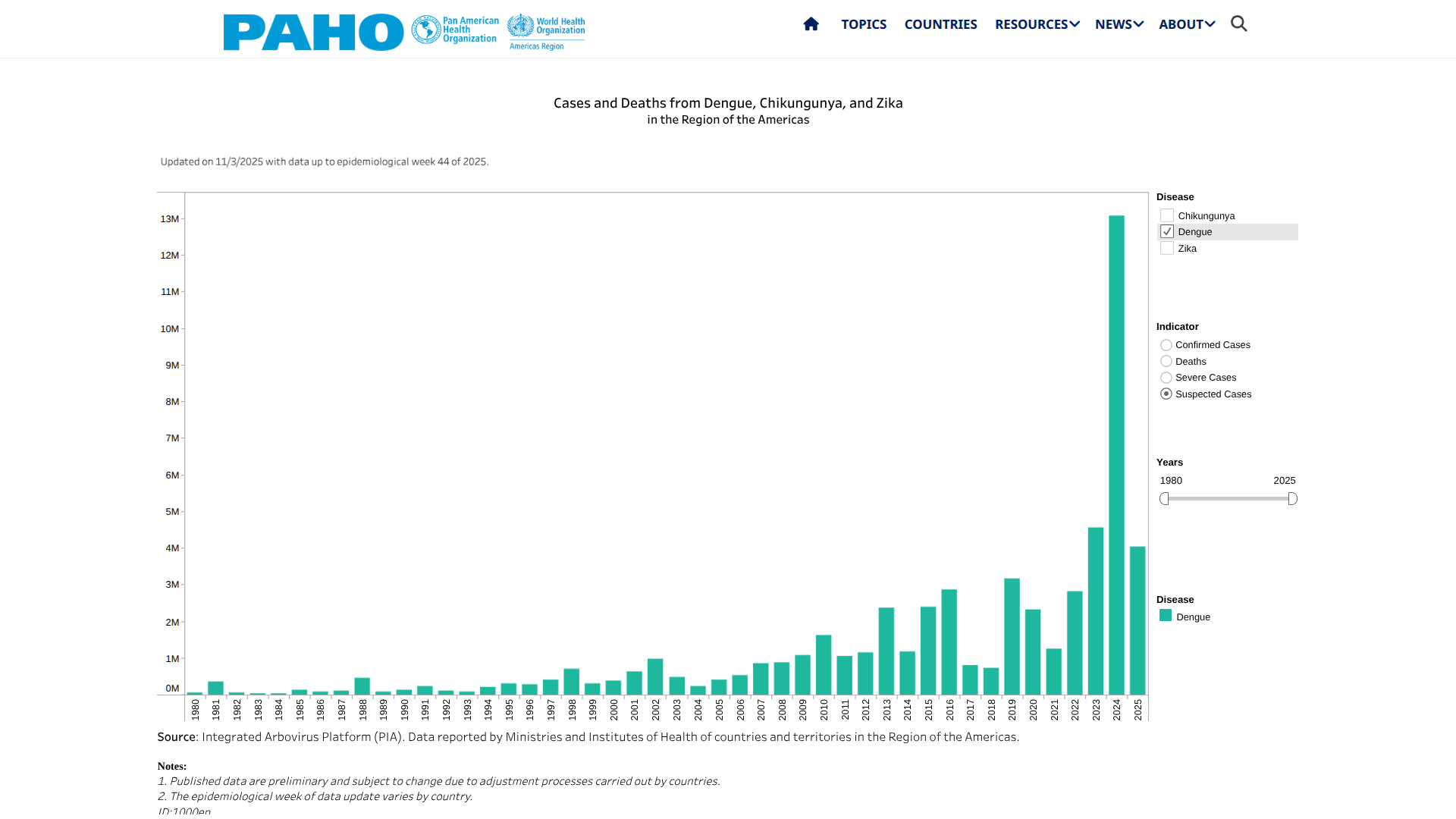
In response to the unexpected spike in Dengue fever cases in the Americas during 2024, the Pan American Health Organization (PAHO) has been committed to transparency in the presentation of data to better inform the international travel community.
The PAHO's efforts are focused on the Southern Cone subregion, which includes Argentina, Brazil, Chile, Paraguay, and Uruguay.
As of November 4, 2025, the PAHO reported a total of 17,689 Dengue cases and 17 related fatalities in the Argentine Republic this year. Dengue cases peaked in May 2025 and decreased from 2024, when over 580,000 cases were confirmed.
In Argentina's Central and Northwest regions, three dengue serotypes —DENV-1, DENV-2, and DENV-3 —have been detected over the past year.
The U.S. CDC says anyone who lives in or travels to an area with risk of dengue is at risk for infection in 2025.
Currently, Argentina is not included in the CDC's Global Health Notice.
The first human cases of the Zika virus (ZIKV) were detected in 1952; researchers have been working to develop a preventive vaccine.
This effort is essential as 31 countries and territories have reported cases of congenital microcephaly and other central nervous system malformations associated with Zika virus infection.
To address the unmet medical need, Valneva SE, a France-based specialty vaccine company, has been conducting phase 1 clinical trials, including its second-generation candidate.
Today, Valneva announced positive results of its current Phase 1 clinical trial (VLA1601-102) investigating the safety and immunogenicity of VLA1601, its second-generation adjuvanted inactivated vaccine candidate against Zika.
As of November 4, 2025, the Company confirmed that two doses of VLA1601 were immunogenic across all five treatment arms investigated (i.e., alumadjuvanted Low, Medium, and High antigen dose; Low with additional adjuvants).
The strongest immune response was observed in the double-adjuvant treatment arms (Low+alum+3M-052-AF and Low+alum+CpG1018) with statistically significantly higher neutralizing antibody titers (Geometric Mean Titers - GMTs) at Day 43 and Day 57 than in the single-adjuvant (alum) treatment arm.
And the immune response induced by the double-adjuvanted VLA1601 was successfully improved compared to the first-generation vaccine candidate, with higher peak seroconversion rates (>93% vs 86%) and peak Geometric Mean Fold Increase in titers (> 56-fold vs > 7-fold).
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release, "We are pleased by the notable safety and immunogenicity results demonstrated for our Zika vaccine candidate and especially our double-adjuvantation results."
Should this vaccine development effort achieve US-FDA approval, international travelers, especially pregnant women, would be very interested in discussing immunization options with healthcare providers. Since 2013, 31 countries and territories, including Costa Rica and Puerto Rico, have reported cases of congenital microcephaly and other central nervous system malformations associated with Zika virus infection.
As of November 4, 2025, over 24,000 Zika cases and four related fatalities have been reported in the Region of the Americas this year.

Since its first approval in Indonesia in 2022, Japan-based Takeda's QDENGA® dengue vaccine has been authorized in 41 countries and distributed in 11 dengue-endemic countries to help reduce the global threat posed by this mosquito-transmitted disease.
Today, Takeda announced very positive vaccine efficacy data.
On November 3, 2025, the company announced the completion of the 7-year pivotal Phase 3 Tetravalent Immunization against Dengue Efficacy Study (TIDES) trial evaluating QDENGA. These data, including an exploratory analysis of a booster dose, confirm the favorable benefit-to-risk profile of QDENGA and that the two-dose regimen provides sustained protection against dengue.
This data is consistent with its approved indications in multiple countries worldwide, which could simplify vaccination schedules and increase adherence.
"QDENGA is the most comprehensively studied dengue vaccine, with more than 60,000 participants globally in the clinical program, and these long-term data highlight the durability of its safety and efficacy profile, across diverse populations worldwide," commented Derek Wallace, M.D., president of the Global Vaccine Business Unit at Takeda, in a press release.
"We are proud to have worked hand-in-hand with the clinical trial participants, collaborators, and investigators whose contributions have been integral to the success of the TIDES trial and played a role in helping us move closer to a dengue-free world."
Takeda stated it continues to invest in post-marketing research through real-world evidence generation and ongoing pharmacovigilance to deepen understanding of the vaccine's safety and impact.
Obtaining access to this market-leading vaccine is essential for states such as Florida, which has reported 53 locally acquired dengue cases in Brevard, Hillsborough, Miami-Dade, and Pasco counties in 2025.
As of today, QDENGA is unavailable in the United States.

According to the World Health Organization, mpox outbreaks remain a global health risk. Since mpox is a vaccine-preventable disease, access to the approved vaccine is essential.
To address this need, Bavarian Nordic A/S, a global vaccine company, recently issued the following clarification regarding the Health Emergency Preparedness and Response Authority (HERA) framework agreement.
The initial order for 750,000 doses of the MVA-BN (JYNNEOS) smallpox/mpox vaccine announced on October 31, 2025, will be delivered in 2026 and is the result of a new joint procurement contract by the European Commission through HERA.
This represents the second order received in 2025, following the earlier award of a contract option from the U. S. Biomedical Advanced Research and Development Authority in the U.S. Department of Health and Human Services, announced in May.
Bavarian Nordic anticipates additional orders for MVA-BN over the course of the following year.
As of November 3, 2025, in the United States, the JYNNEOS vaccine is commercailly available at various health clinics and pharmacies.

The Public and Environmental Health Office at Colorado State University (CSU) has reported an unusual increase in pertussis cases this semester, with 14 confirmed cases in the fall 2025 semester.
According to a CSU media statement on October 30, 2025, pertussis, known as whooping cough, is a highly contagious bacterial infection that spreads through respiratory droplets when an infected person coughs or sneezes.
CSU students are encouraged to contact the Health Network's Immunizations Department to verify whether they are up to date with their TDAP vaccine. The health department emphasizes that vaccination is the best defense against this infection.
Located in Fort Collins, 65 miles north of Denver, a community of about 1 million, CSU has an enrollment of 33,000.
In Colorado, the TDAP vaccine is recommended by the Health Department for most students and residents.