First Rapid Antigen Tests Prequalified for COVID-19
The World Health Organization (WHO) recently announced a significant milestone: the prequalification of the first two rapid antigen diagnostic tests for SARS-CoV-2, the respiratory virus that causes the disease known as COVID-19.
This is the first instance where these rapid antigen tests have received full prequalification from WHO, ensuring their quality, safety, and performance over the long term.
Announced on December 17, 2025, the approved tests are the SD Biosensor STANDARD Q COVID-19 Ag Test and the ACON Biotech Flowflex SARS-CoV-2 Antigen Rapid Test.
These tests provide results within 15 to 30 minutes, are cost-effective, and can be used in clinics, communities, or at home. This enables rapid identification of infectious cases and supports public health responses in conjunction with molecular testing.
WHO prequalification elevates these tests beyond emergency status, making them eligible for procurement by UN agencies, global health partners, and governments.
This move is expected to enhance access in low- and middle-income countries through pooled procurement, lower prices, and stable supply chains, effectively addressing barriers like cost and regulatory challenges.
The CDC says, 'COVID-19 testing can help you know if you have COVID-19 so you can decide what to do next, like getting treatment to reduce your risk of severe illness and taking steps to lower your chances of spreading the virus to others.'
'Nucleic acid amplification tests (NAATs), including PCR tests, are more likely to detect the virus than antigen tests.'
'NAATs tests are the "gold standard" for COVID-19 tests,' confirms the CDC.
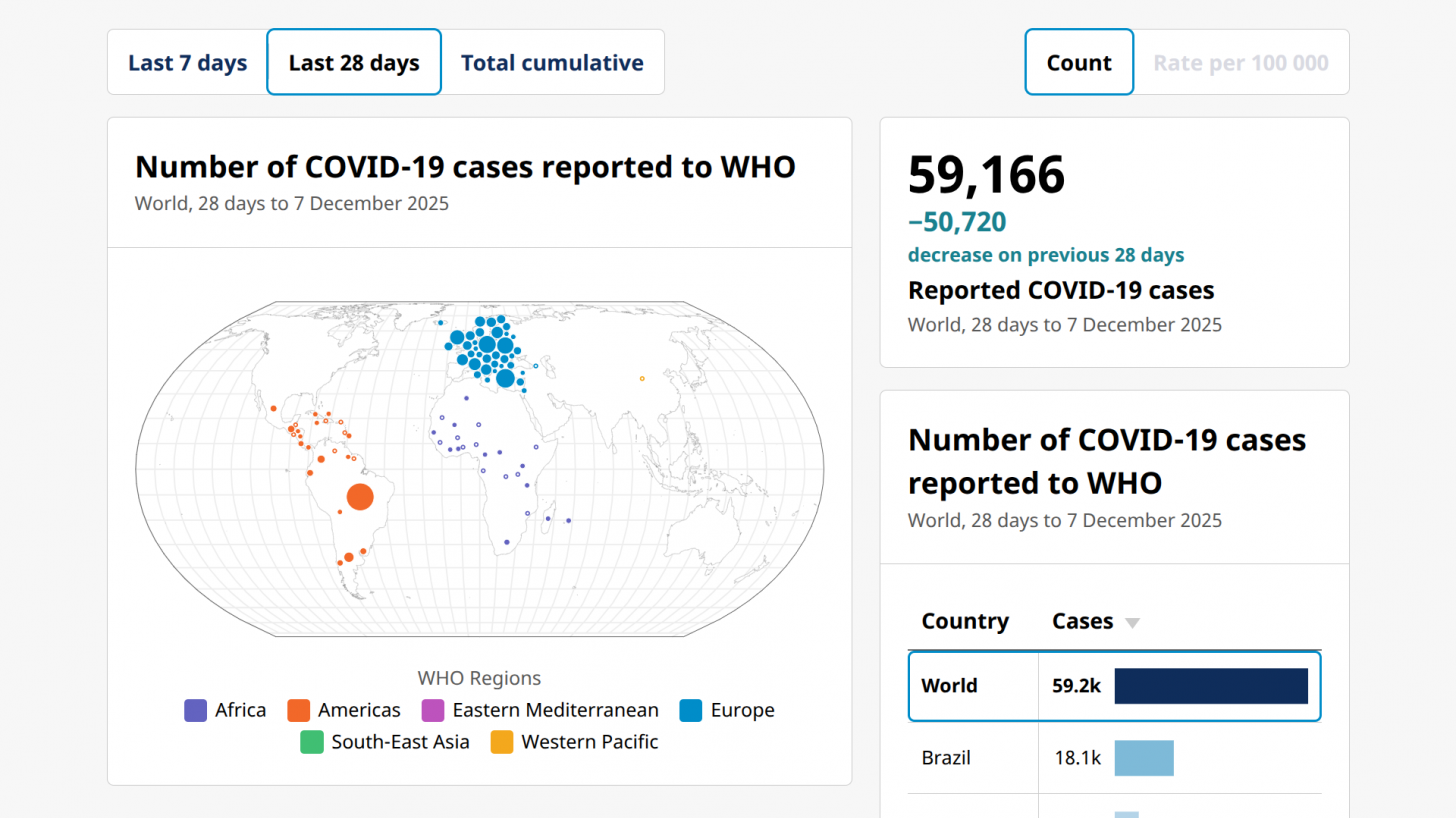
More than two years after the WHO ended the COVID-19 public health emergency in May 2023, the virus continues to circulate globally and in the United States.
As of early December, the WHO data indicated a significant COVID-19 outbreak in western Europe.
In the U.S., COVID-19 has been detected in various states across the U.S. Central region, as of December 31, 2025.
Our Trust Standards: Medical Advisory Committee
























