South Asia Combats Nipah Virus Infections with Innovative Vaccine Candidate
The World Health Organization (WHO) is closely monitoring the situation of Nipah virus (NiV) infections in South Asia, where the virus continues to pose a public health challenge despite strong containment efforts by national authorities.
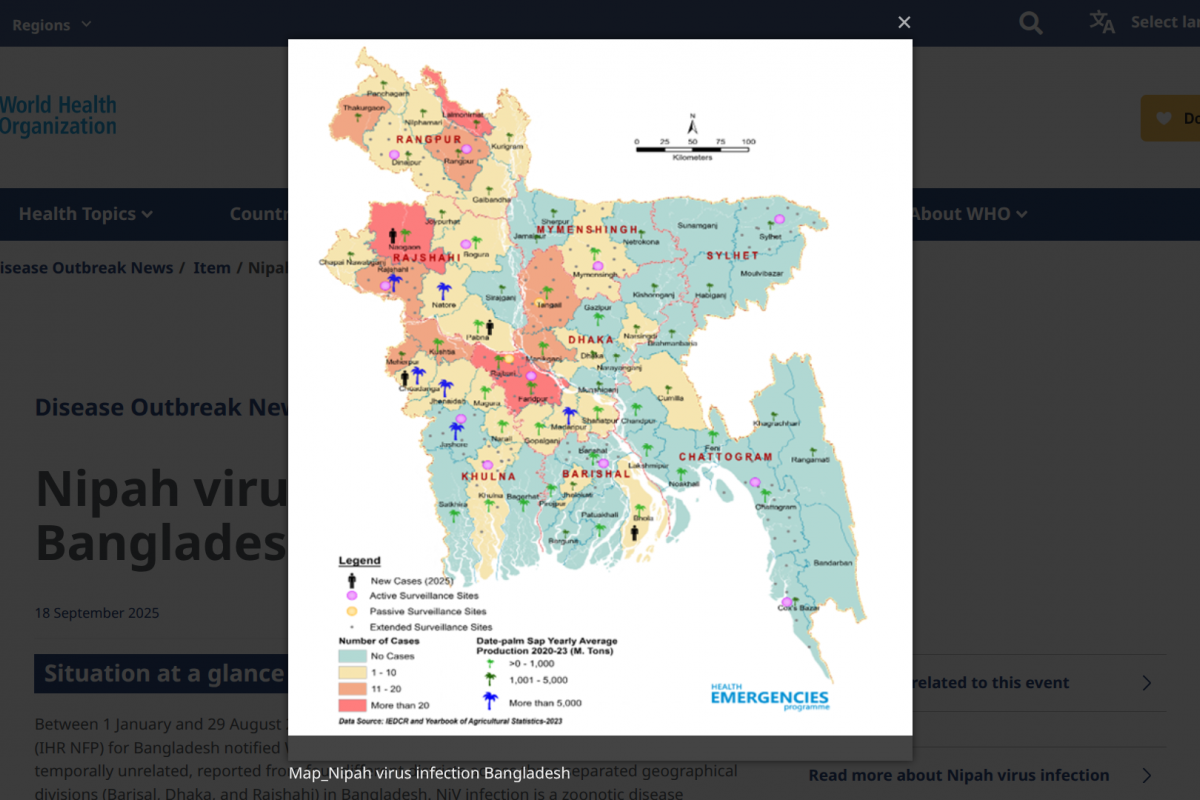
First identified after an outbreak in Malaysia, an outbreak occurred in Bangladesh in 2001, and human NiV cases have been confirmed nearly every year in the country.
To date, the WHO Disease Outbreak News (577) reports that Bangladesh has documented 347 cases through its established surveillance system, with a case fatality rate of 71.7%.
In 2025, Neighboring India reported four confirmed NiV cases in Kerala state, including two deaths, across Malappuram and Palakkad districts—the first recorded cases in Palakkad.
Kerala's health authorities, through the Information and Public Relations Department, promptly issued alerts, traced contacts, and intensified containment measures, preventing wider spread.
The WHO currently assesses the public health risk from NiV as moderate at national and regional levels, given the high fatality rate and challenges in early detection.
However, the risk of international spread remains low, with no confirmed cases outside the affected areas in Asia.
Furthermore, the WHO advises travelers and residents in affected regions to avoid raw date palm products during the season and to seek immediate medical care for symptoms such as fever, headache, or respiratory issues.
Unfortunately, as of December 27, 2025, the WHO reports that no specific drugs or vaccines exist.
In a significant advancement against the deadly virus, the University of Oxford announced on December 15, 2025, that it has launched the world's first Phase II clinical trial of its ChAdOx1 NipahB vaccine candidate in Bangladesh.
In recognition of the urgent need for a Nipah virus vaccine and the compelling early data, the European Medicines Agency granted the ChAdOx1 NipahB vaccine PRIME designation in June 2025. This designation aims to expedite the development and regulatory review processes for medicines that address unmet medical needs.
The ChAdOx NipahB vaccine was manufactured for this clinical trial by the Serum Institute of India Pvt. Ltd., the world's largest vaccine manufacturer, in collaboration with the Coalition for Epidemic Preparedness Innovations.
Vax-Before-Travel lists additional Nipah virus vaccine candidates.
Our Trust Standards: Medical Advisory Committee