Mpox / Smallpox Vaccine Studied for Infants, Breastfeeding and/or Pregnant Women

With the continued confirmations of mpox infections in various countries, the U.S. FDA-approved vaccine is now being evaluated for at-risk, vulnerable populations.
Bavarian Nordic A/S announced that on June 26, 2025, the initiation of the first of two clinical trials designed to support approval and use of the MVA-BN® (JYNNEOS) mpox/smallpox vaccine in infants under 2 years of age, pregnant, and breastfeeding women.
Both studies are conducted in the Democratic Republic of Congo, the epicentre of the ongoing mpox outbreak in Africa, where infants and pregnant women remain highly vulnerable to the sexually transmitted mpox virus.
Paul Chaplin, President & CEO of Bavarian Nordic, commented in a press release, “These new studies will fill the gap by providing important data about the use of MVA-BN ... which could help support a label expansion for MVA-BN to include the most vulnerable populations.”
Furthermore, these studies are part of the PregInPoxVac research project, which includes a phase 2 trial of MVA-BN in children aged 2-11 years. Topline results from this trial (NCT06549530) are anticipated in the third quarter of 2025.
Currently, JYNNEOS is commercially offered in the United States at various clinics and pharmacies.
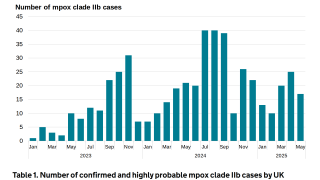
According to the U.S. CDC, the majority of clade II mpox cases in the U.S. continue to be in people who are not vaccinated or who have received only one dose of JYNNEOS.
As of June 1, 2025, approximately 35,000 mpox infections had been reported in the United States.
Our Trust Standards: Medical Advisory Committee