Positive Phase 2 Data for Lyme Disease Vaccine Candidate Confirmed

New York-based Pfizer Inc. and Valneva SE today announced further positive Phase 2 data for their Lyme disease vaccine candidate, VLA15, the only Lyme disease vaccine candidate currently in clinical development.
Valneva and Pfizer plan to proceed with a three-dose primary series vaccination schedule in a planned Phase 3 clinical trial based on these new results.
The trial will evaluate VLA15 in adults and pediatric subjects five years of age and above and is expected to be initiated in 2022, subject to regulatory approval.
The ongoing Phase 2 clinical trial, VLA15-221, compared the immunogenicity of VLA15 after administration of two (at months 0 and 6) or three (at months 0, 2, and 6) primary series doses in groups aged 5-11, 12-17, and 18-65 years.
Initial pediatric data are expected in the first half of 2022.
In the sub-analysis of adult participants (18-65 years old) who received VLA15 in either the two-dose schedule (N=90) or the three-dose schedule (N=97), performed one month after the last vaccination dose, VLA15 was found to be immunogenic with both vaccination schedules tested.
The analysis was also consistent with the acceptable safety and tolerability profile observed in previous studies of VLA15. In addition, no vaccine-related serious adverse events (SAEs) were observed.
These data are consistent with the strong immunogenicity profile observed for this age group in previous Phase 2 studies.
However, the induction of anti-OspA IgG (anti-outer surface protein A immunoglobulin G) antibody titers was higher in participants who received the three-dose primary series compared to those who received the two-dose primary series, supporting the use of a three-dose primary series schedule in the planned Phase 3 clinical trial.
Kathrin U. Jansen, Ph.D., SVP, and Head of Vaccine Research & Development at Pfizer, commented in a Valneva press release issued on February 4, 2022, "Lyme disease is increasingly impacting people throughout the northern hemisphere, potentially due to environmental changes and more active outdoor lifestyles."
"The continued positive data from the VLA15-221 trial support the ongoing development of this vaccine candidate, and we look forward to continuing to work with Valneva to potentially help protect people against Lyme disease."
This investigational multivalent protein subunit VLA15 vaccine uses an established mechanism of action for a Lyme disease vaccine that targets the outer surface protein A (OspA) of Borrelia burgdorferi, the bacteria that cause Lyme disease.
OspA is one of the most dominant surface proteins expressed by the bacteria when present in a tick.
The VLA15 vaccine covers the six OspA serotypes expressed by Borrelia burgdorferi sensu lato species prevalent in North America and Europe.
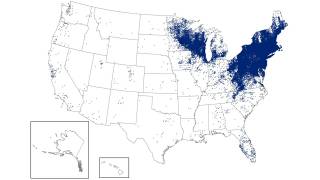
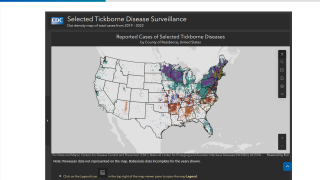
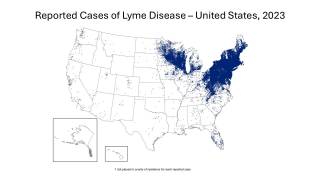
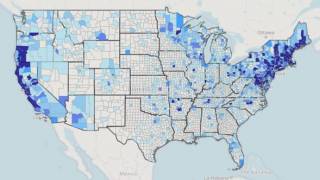
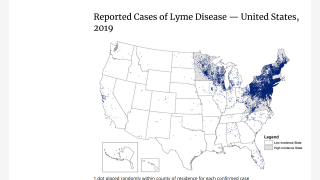
Lyme disease is the most common vector-borne disease in the United States, stated the U.S. CDC. Lyme disease is caused by the bacterium Borrelia burgdorferi and, rarely, Borrelia mayonii.
It is transmitted to humans through the bite of infected blacklegged ticks.
The states of Pennsylvania, New Jersey, and New York have reported the most recent cases.
The CDC also reports Lyme disease cases by age, which indicates most cases in the U.S. are reported by seniors over the age of 55.
The program was granted Fast Track designation by the U.S. FDA in July 2017.
Valneva and Pfizer entered into a collaboration agreement in April 2020 to co-develop the VLA15 vaccine.
Valneva is a specialty vaccine company based in Saint-Herblain, France, focused on the development and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical need.
Our Trust Standards: Medical Advisory Committee