Will Herpes Vaccines Follow Gonorrhea in 2026

Global public health efforts have achieved notable advancements in 2025 in preventing sexually transmitted infections (STIs), even as over one million new cases are acquired daily.
According to the World Health Organization (WHO), vaccinations have emerged as a critical tool, particularly for human papillomavirus (HPV) and Hepatitis B.
HPV vaccinations dominated public interest and utilization throughout 2025, driven by international campaigns and initiatives to eliminate HPV-related cancers such as cervical cancer.
Additionally, Hepatitis B vaccination remained a staple in routine immunization schedules, providing longstanding protection against this viral STI and its associated liver complications, says the WHO.
And in 2025, a groundbreaking development came from the United Kingdom, which in August became the first country to launch a targeted gonorrhea prevention program using the meningococcal B vaccine 4CMenB (Bexsero).
This off-label initiative leverages 30-40% cross-protection against Neisseria gonorrhoeae, owing to bacterial similarities to neisseria meningitis.
The UK's effort will provide health policy experts with essential insights, as no dedicated gonorrhea vaccine reached approval in 2025, and prospects for 2026 appear inconsequential.
For example, a University of California Health randomized, observer-blind, placebo-controlled, multi-site phase 2 clinical trial of Bexsero launched in 2020 is expected to be completed around February 26, 2026.
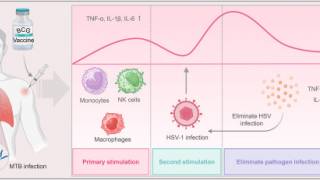
At the same time, innovative approaches addressing herpes are in demand amid growing antimicrobial resistance.
In late December, Gilead Sciences, Inc. announced that it had exercised its option to exclusively license two investigational herpes therapeutic candidates from Assembly Biosciences, Inc.
Additionally, following the discontinuation of competitors' vaccine programs, BioNTech SE's mRNA-based preventive vaccine candidate (BNT163) is currently the most prominent active candidate. Its Phase 1 clinical trial, BNT163-01, is estimated to be completed by October 2026.
As of December 29, 2025, public interest in a HSV-1/HSV-2 vaccine remains notably high among young adults.
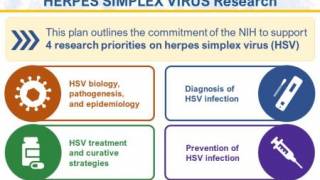
Global vaccine researchers, including Vax-Before-Travel.com, report that herpes vaccine news consistently ranked among the most-requested topics among readers throughout 2025. This digital news engagement is evident in the WHO's call for increased investment in HSV prevention tools.
Industry researchers at the U.S. NIH say widespread herpes vaccine availability remains unlikely before the early 2030s, as advancing through Phases 2 and 3 clinical trials reviewed by the refocused U.S. FDA will require years.
With 2026 on the horizon, these vaccines offer optimism for curbing the global STI burden in the decade ahead.
Our Trust Standards: Medical Advisory Committee