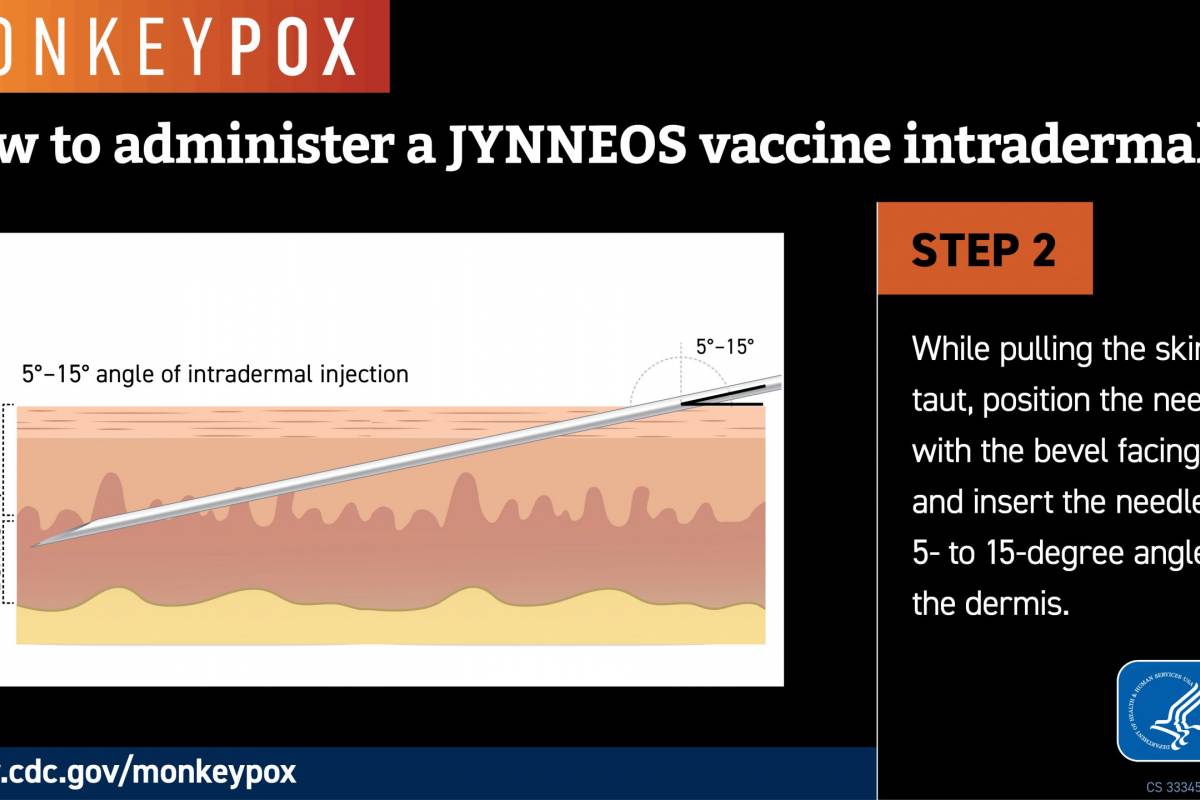
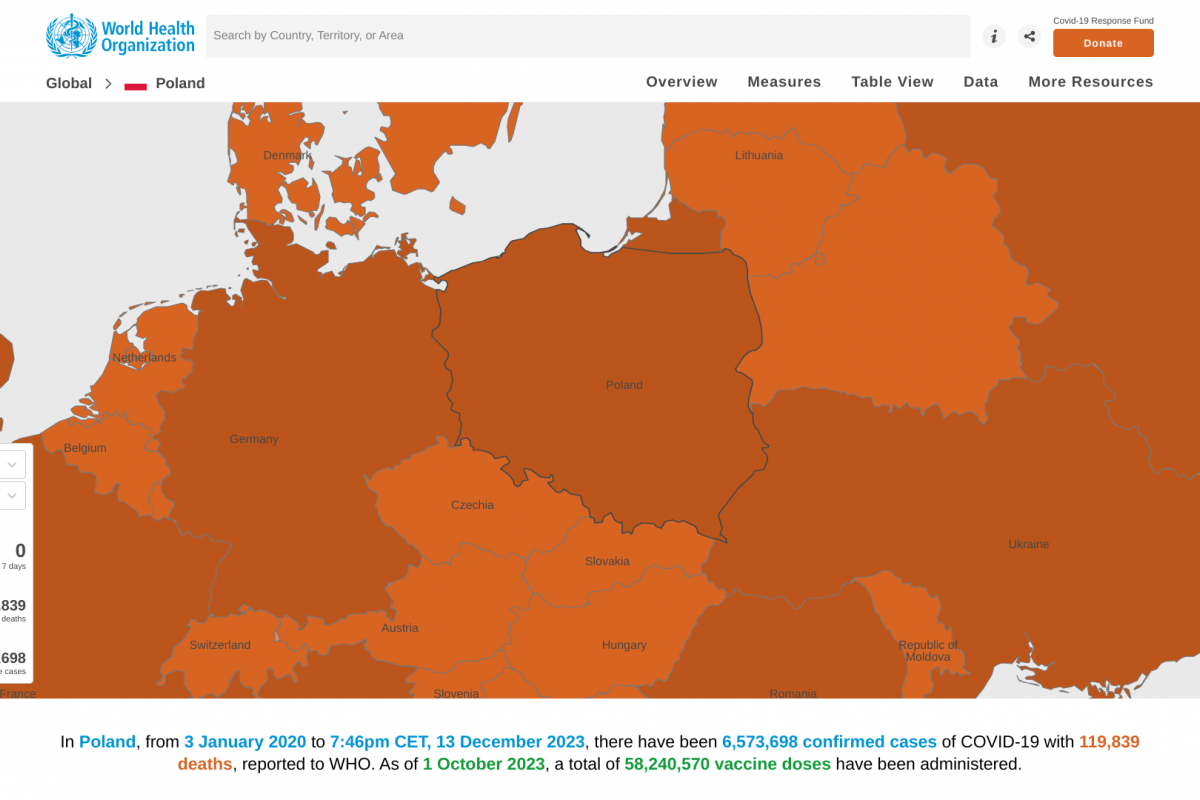
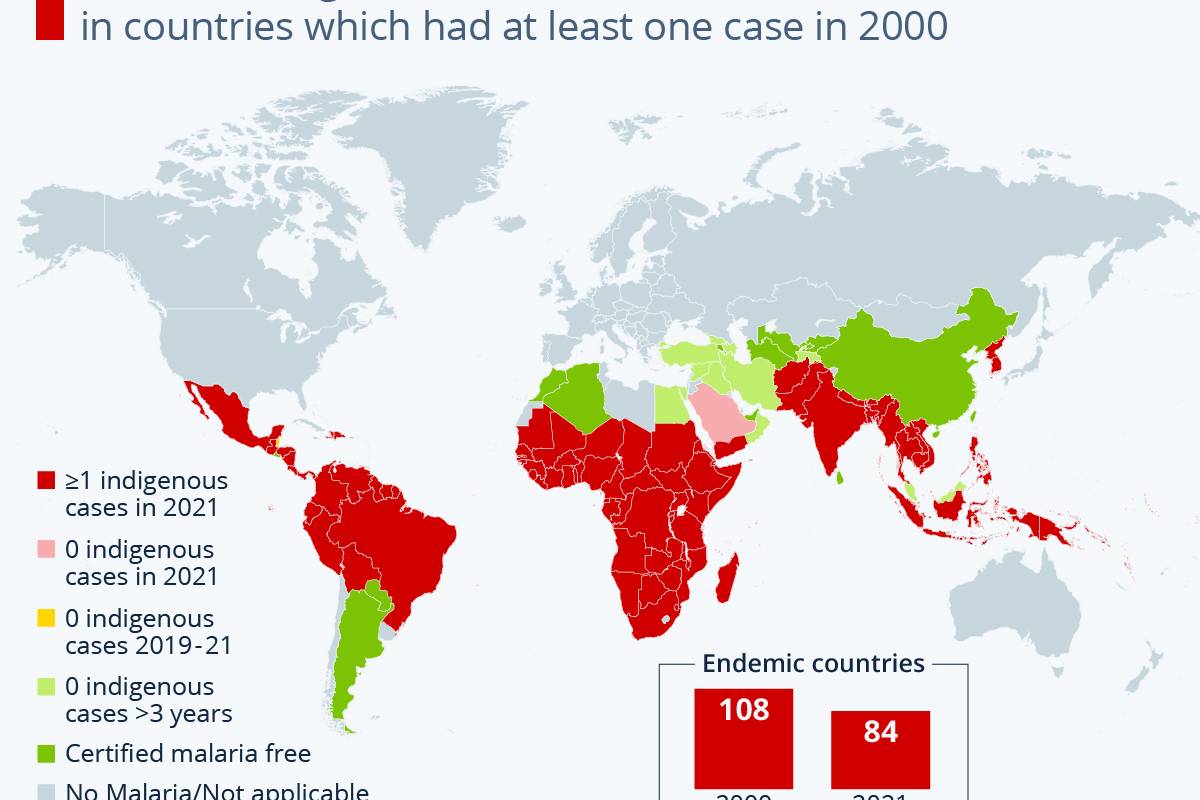
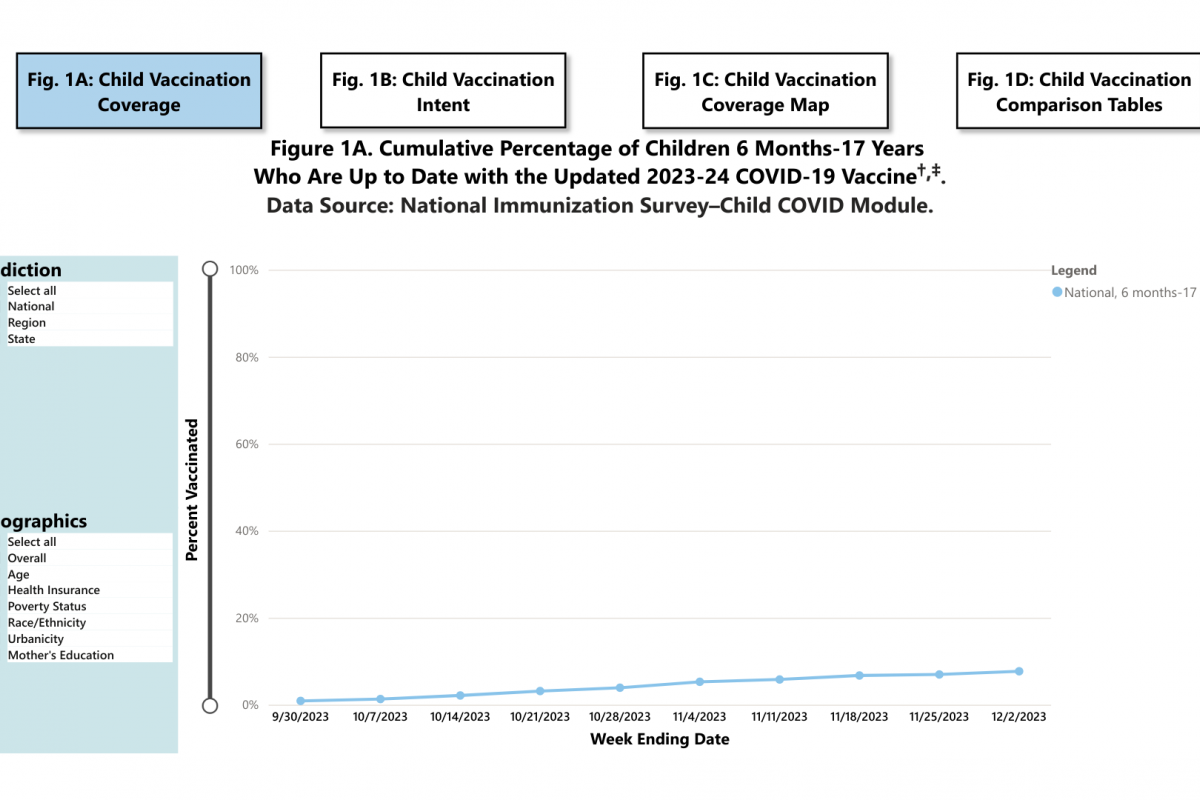
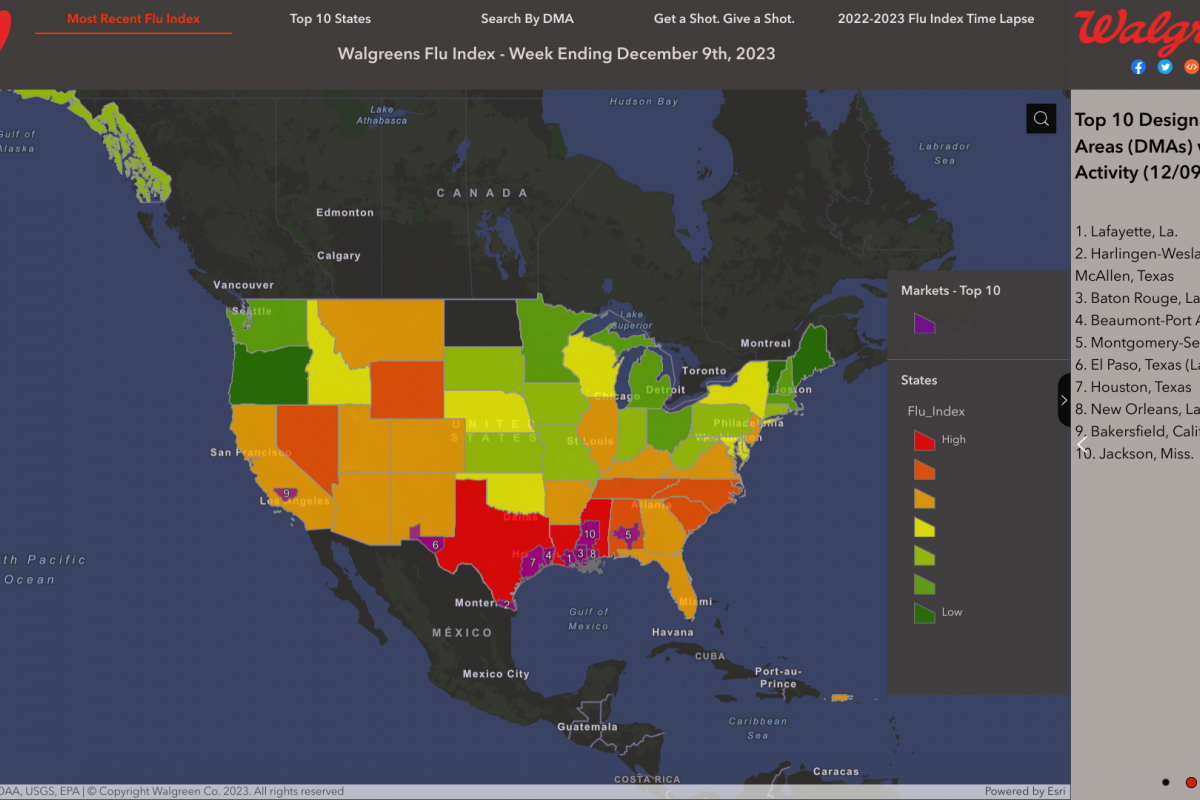
Sanofi and AstraZeneca recently announced they continue to engage with government officials, healthcare providers, and others in the United States regarding the supply of Beyfortus™ (nirsevimab-alip), a respiratory syncytial virus (RSV) monoclonal antibody therapy.
On December 14, 2023, the companies wrote, Since we launched in September 2023, hundreds of thousands of infants in the U.S. have received Beyfortus: 50 mg or 100mg Injection.
This is the first season in which passive immunization for babies against RSV has been provided broadly.
By the end of January 2024, a total of 1.4 million babies will be offered protection against RSV, a 27% increase over the initial supply forecast for the 2023-2024 RSV season in the U.S.
Beyfortus is a prescription injectable medicine used to help prevent a serious lung disease caused by RSV in babies under one year of age born during or entering their first RSV season and for children up to 24 months of age who remain at risk of severe RSV disease through their second RSV season.
It is a recombinant human immunoglobulin G1 kappa (IgG1ĸ) long-acting mAbs that binds to the prefusion conformation of the RSV F protein.
The most common side effects of Beyfortus include rash and pain, swelling, or hardness at the site of your child’s injection.
On October 23, 2023, the U.S. CDC published Interim Recommendations to Protect Infants from RSV (CDCHAN-00499) during the 2023–2024 RSV season. RSV antibody therapy was initially authorized in the U.S. in 1998.