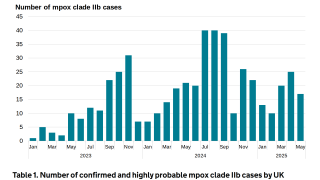
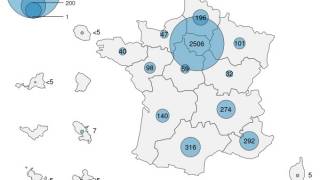
Smaller Mpox Vaccine Doses Found Effective Between the Skin
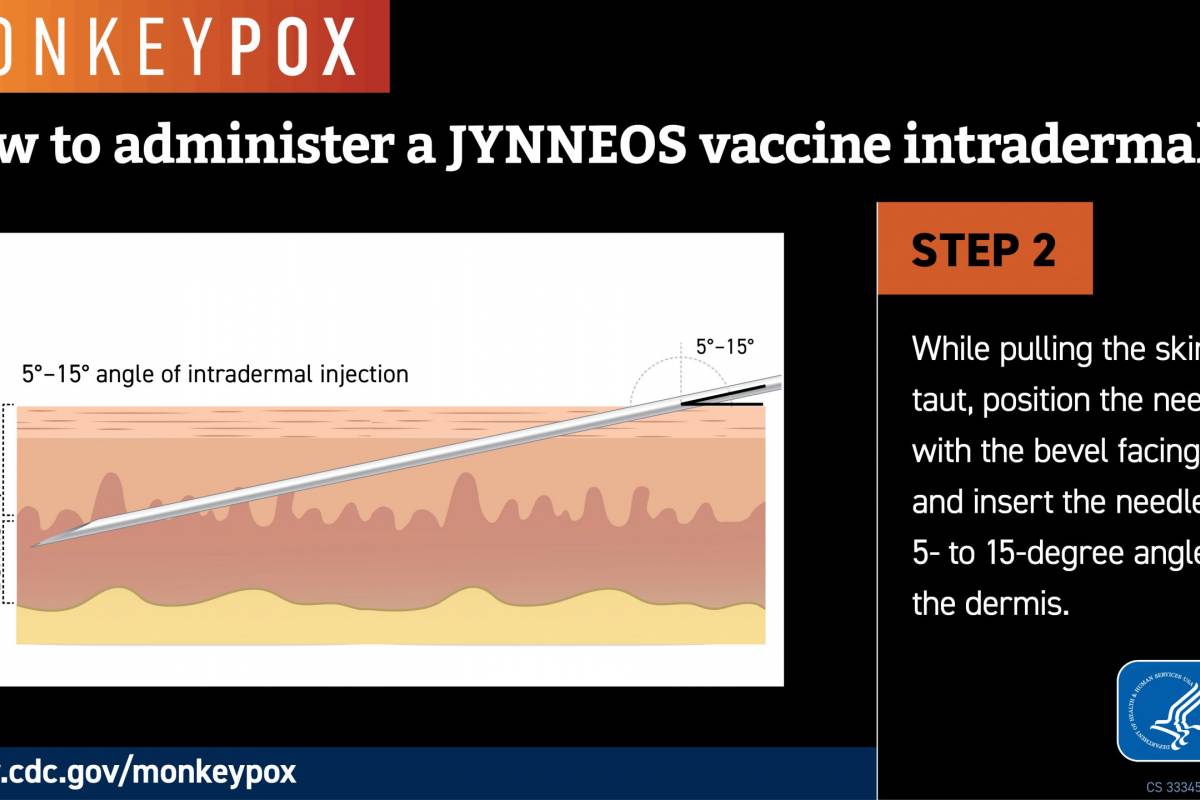
Led by researchers at NYU Grossman School of Medicine, a recent study showed no significant difference in the strength of the immune response (IgG antibodies) between most of those who received their vaccine injections in small doses between layers of the skin.
In some cases, the IgG antibodies were detected more than six months after the second and final JYNNEOS® vaccination.
Because of limited space between skin layers, intradermal injections can only accommodate small doses, while larger doses generally require subcutaneous injections.
The smaller doses, about one-fifth of the usual full dose and spread out by as long as three months, were authorized by the U.S. FDA and CDC in August 2022.
About 155,000 New Yorkers have been vaccinated, mainly using smaller doses.
"Our study shows that smaller vaccine doses of mpox vaccine administered in two doses spread out over weeks to months were similar to the full (subcutaneous) FDA-approved dose," said study co-lead investigator and infectious disease specialist Angelica Cifuentes Kottkamp, MD, in a press release on December 14, 2023,
"Implementing the smaller dose was a good emergency measure in the face of immediate shortages of the vaccine," said Dr. Kottkamp, an assistant professor in the Department of Medicine at NYU Langone Health.
Additionally, the New England Journal of Medicine published a Correspondence that revealed people fully vaccinated with two smaller JYNNEOS doses had an immune response four times stronger than those who did not complete the vaccination series and had only one dose.
This study's finding is significant since The Lancet Infectious Diseases reported on December 7, 2023, that 12% of JYNNEOS vaccinated individuals were non-antibody responders.
Bavarian Nordic codeveloped JYNNEOS with the U.S. Government to ensure adult populations, including people with weakened immune systems, could be protected from smallpox.
On February 22, 2023, the U.S. CDC issued Interim Clinical Considerations confirming that mpox vaccination should continue to be offered to people with the highest potential for exposure to mpox. The U.K. and Europe have issued similar notices in 2023.
Our Trust Standards: Medical Advisory Committee