Bavarian Nordic A/S, a leading pharmaceutical company, announced news today regarding its investigational chikungunya vaccine, CHIKV VLP.
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) has granted accelerated assessment for this chikungunya vaccine candidate's Marketing Authorisation Application (MAA).
The CHMP has recognized that the vaccine candidate is of significant interest to public health and therapeutic innovation.
With this positive development, the company is taking steps toward addressing the unmet medical needs of millions worldwide affected by the Chikungunya virus.
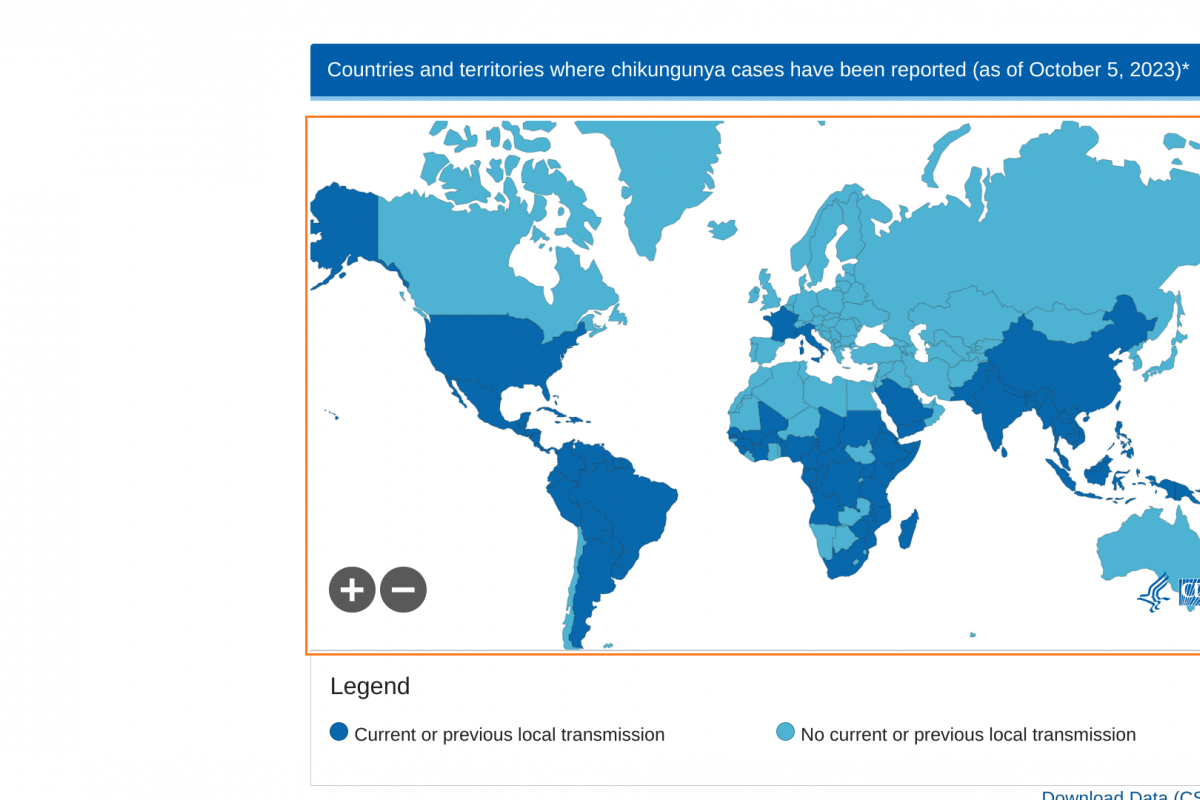
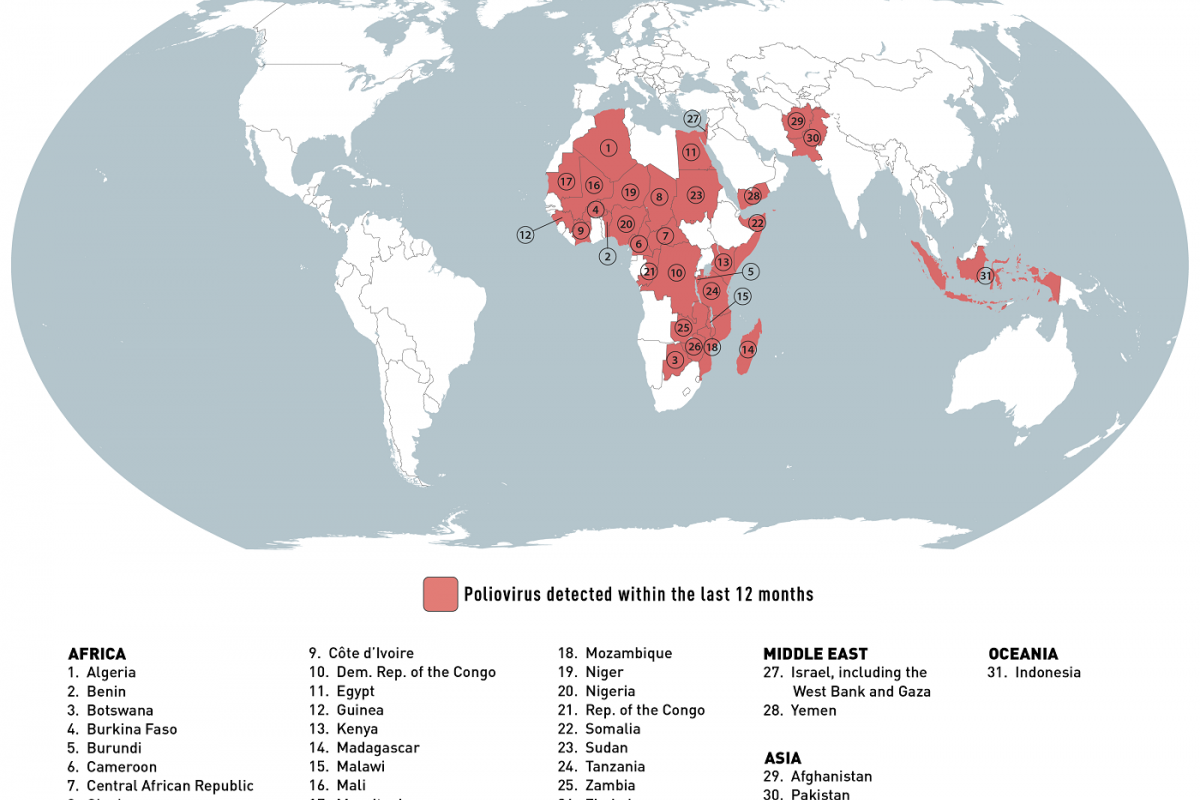
Chikungunya outbreaks continue to be reported in 2024.
Before 2013, chikungunya virus cases and outbreaks had been identified in countries in Africa, Asia, Europe, and the Indian and Pacific Oceans. In late 2013, the first local transmission of chikungunya virus in the Americas was identified in Caribbean countries and territories, according to the U.S. CDC.
Bavarian Nordic also confirmed on February 23, 2024, that it is on track to submit its MAA for CHIKV VLP to the EMA during H1 2024. As a result, the review of the MAA may now take as little as 150 days instead of the usual 210 days.
This means that the vaccine could be available in Europe sooner than expected.
"We are pleased to receive the accelerated assessment in recognition of our chikungunya vaccine candidate and our efforts to bring this novel product to the market. With this, we can accelerate the approval and launch timelines for the vaccine in Europe. As part of our global strategy, we also plan to submit our biologics license application (BLA) for the vaccine candidate to the U.S. Food and Drug Administration later this year," said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release.
In 2023, Bavarian Nordic successfully completed two Phase 3 studies of CHIKV VLP.
The CHKV-VLA vaccine candidate received the U.S. Food and Drug Administration (FDA) Fast Track designation in May 2018.
Recently, the FDA issued approval for Valneva SE's IXCHIQ® Chikungunya Vaccine. However, the CDC has not given its approval.