The U.S. CDC's Weekly COVID-19 Vaccination Dashboard estimates vaccinations and intent for vaccination using various data sources, including surveys, healthcare claims, electronic medical records, and immunization information systems data.
As of December 6, 2023, category highlights include the following data:
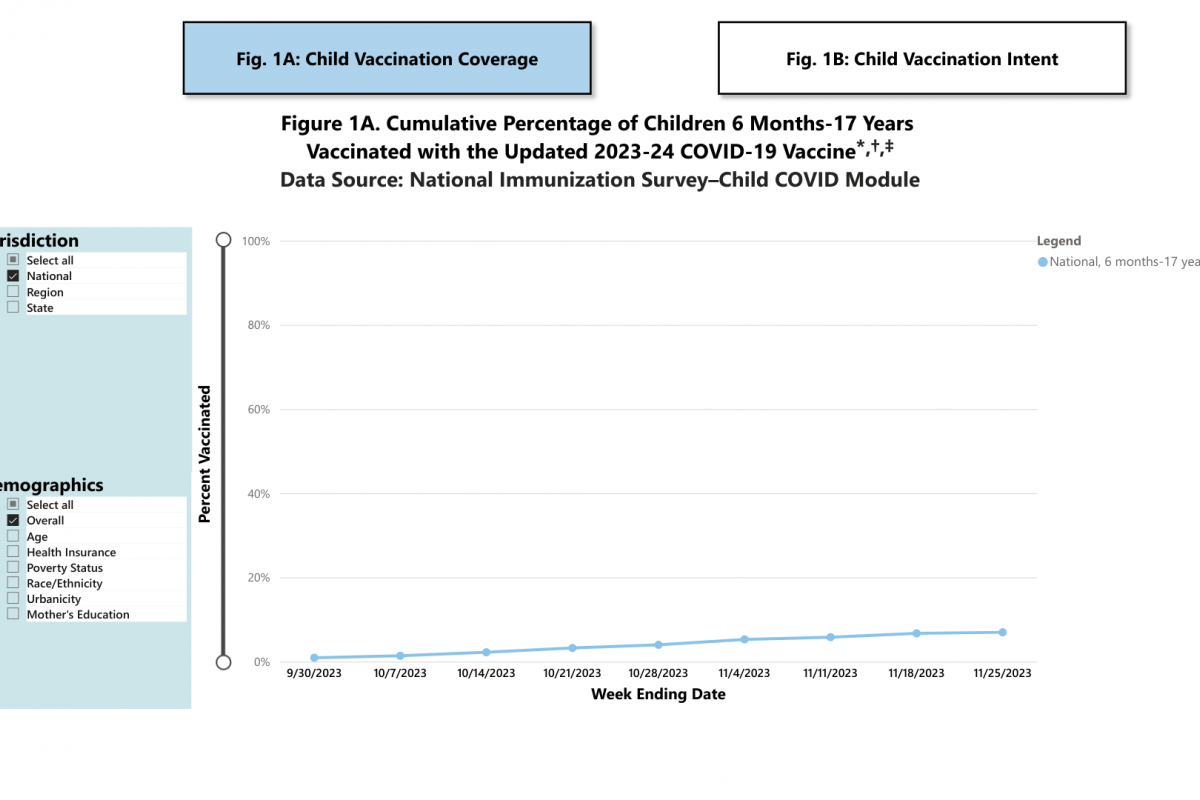
As of November 25, 2023, 6.9% (95% Confidence Interval: 6.0%-7.9%) of children were reported to be up-to-date with the 2023-24 COVID-19 vaccine.
About 17.3% of children had a parent who reported they planned to get their child vaccinated. Additional COVID-19 vaccination data for children by demographic characteristics at the national level and overall estimates by jurisdiction are available at this CDC link.
For pregnant women, 8.9% had received the updated 2023-24 COVID-19 vaccine.
Vaccination coverage was highest among non-Hispanic Asian (15.2%) women and lowest among non-Hispanic Black (2.7%) pregnant women.
And for adults, about 16% reported having received an updated 2023-24 COVID-19 vaccine since September 14, 2023. Vaccination coverage increased by age and was highest among adults 65 and older [33.3%, (31.2%-35.3%)].
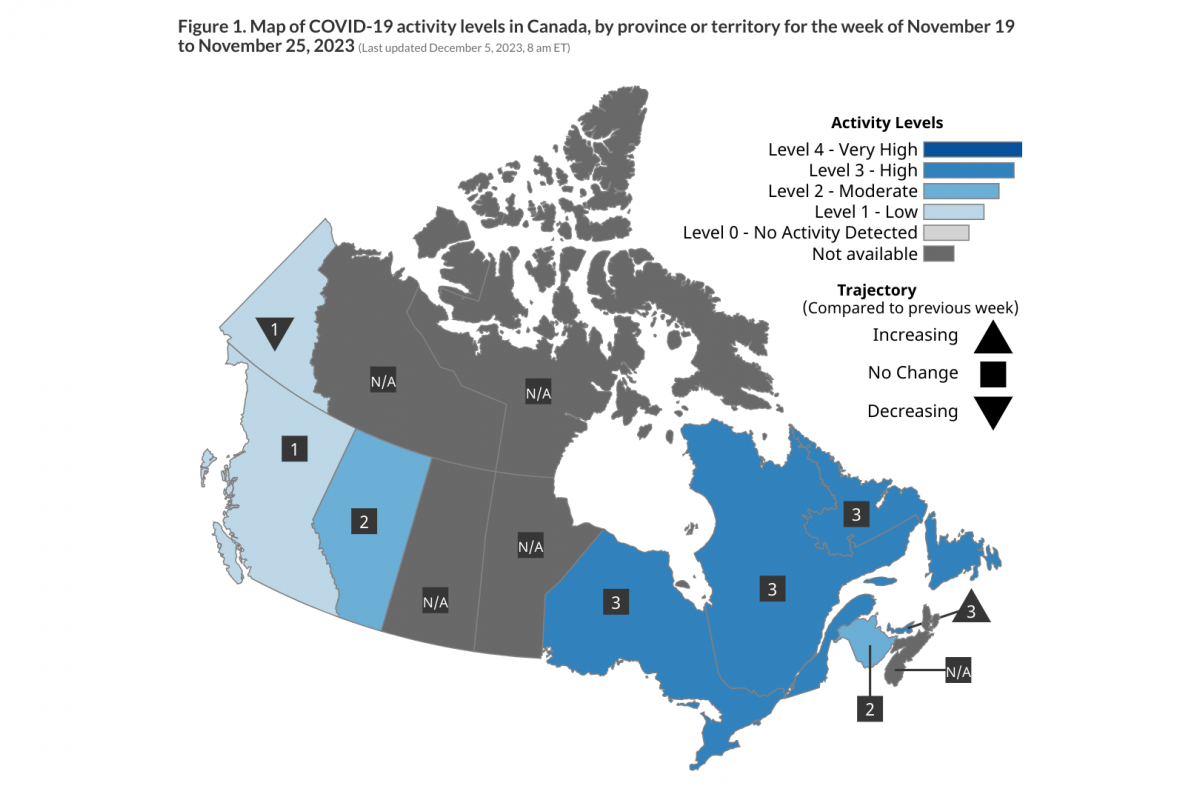
From a geography perspective, the District of Columbia reported the most significant number of vaccinated adults, with about 30.7%.
COVID-19 vaccination coverage estimates among all adults are based on CDC's National Immunization Survey–Adult COVID Module data.