Canada Authorized Enhanced Protein-Based COVID-19 Vaccine
Novavax, Inc. today announced that Health Canada has granted expanded authorization for Nuvaxovid™ XBB.1.5 Vaccine (Recombinant protein, Adjuvanted) for active immunization to prevent COVID-19 caused by the SARS-CoV-2 coronavirus in individuals aged 12 and older.
The Public Health Agency of Canada's National Advisory Committee on Immunization recommended XBB COVID-19 vaccines that target more recent, immune-evasive virus variants.
The expanded authorization was based on non-clinical data showing that Novavax's COVID-19 vaccine induced functional immune responses against XBB.1.5, XBB.1.16, and XBB.2.3 variants.
Additional non-clinical data demonstrated that Novavax's vaccine-induced neutralizing antibody responses to subvariants BA.2.86, EG.5.1, FL.1.5.1, and XBB.1.16.6 as well as CD4+ polyfunctional cellular (T-cell) responses against EG.5.1 and XBB.1.16.6.
These data indicate that Novavax's vaccine can stimulate both arms of the immune system and may induce a broad response against currently circulating variants.
In Canada, recombinant XBB sub-lineages remain dominant, representing 93% of sequences in the past month. HV.1, HK.3, and BA.2.86 (including JN.1) are the major lineage groups demonstrating consistent growth in Canada.
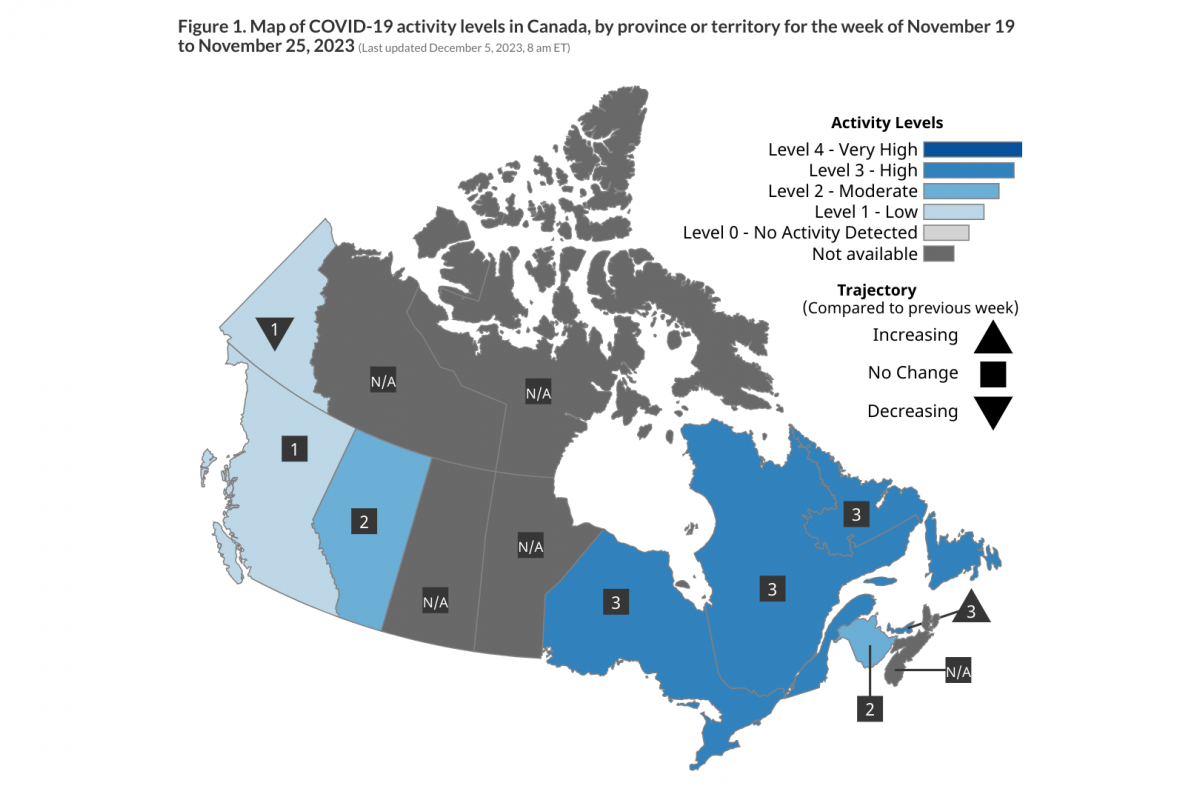
Health Canada's latest COVID-19 numbers were updated at this link as of December 5, 2023, 8 am ET.
"Today's expanded authorization will support the Canadian government's strong commitment to provide its citizens with effective options, such as our protein-based non-mRNA vaccine, in the campaign against currently circulating COVID-19 variants," said John C. Jacobs, President and Chief Executive Officer, Novavax, in a press release on December 5, 2023.
Novavax's updated COVID-19 vaccine is also authorized in the U.S. and Europe by the World Health Organization and is under review in other markets.
Our Trust Standards: Medical Advisory Committee























