The current outbreaks of avian influenza ("bird flu") continue to cause devastation in animal populations worldwide. Although largely affecting animals, these outbreaks pose ongoing risks to humans, says the World Health Organization (WHO).
The WHO wrote today that an increasing number of H5N1 avian influenza detections among mammals, which are biologically closer to humans than birds, raises concern that the virus might adapt to infect humans more easily.
In addition, some mammals may act as mixing vessels for influenza viruses, leading to the emergence of new viruses that could be more harmful to animals and humans.
Announced on July 12, 2023, the WHO, Food and Agriculture Organization of the United Nations, and the World Organisation for Animal Health urge countries to work together across sectors to save as many animals as possible and protect people.
Sporadic influenza A(H5N1) clade 2.3.4.4b virus detections in humans have been reported but remain very rare, with 8 cases reported since December 2021.
But only one case in the U.S.
Infections in humans can cause severe disease with a high mortality rate, says the WHO.
The human cases detected thus far are mostly linked to close contact with infected birds and contaminated environments.
"With the information available so far, the virus does not appear to be able to transmit from one person to another easily, but vigilance is needed to identify any evolution in the virus that can change that," said Dr. Sylvie Briand, Director of Epidemic and Pandemic Preparedness and Prevention, WHO, in a related press release.
"We encourage all countries to increase their ability to monitor these viruses and to detect any human cases. This is especially important as the virus now affects countries with limited prior experience in avian flu surveillance."
Studies are underway to identify any changes in the bird flu virus that may help the virus to spread more easily among mammals, including humans, says the WHO.
The U.S. CDC's Situation Summary issued as of July 5, 2023, confirmed the current risk to the public from bird flu viruses remains low. However, continued sporadic human infections are anticipated.
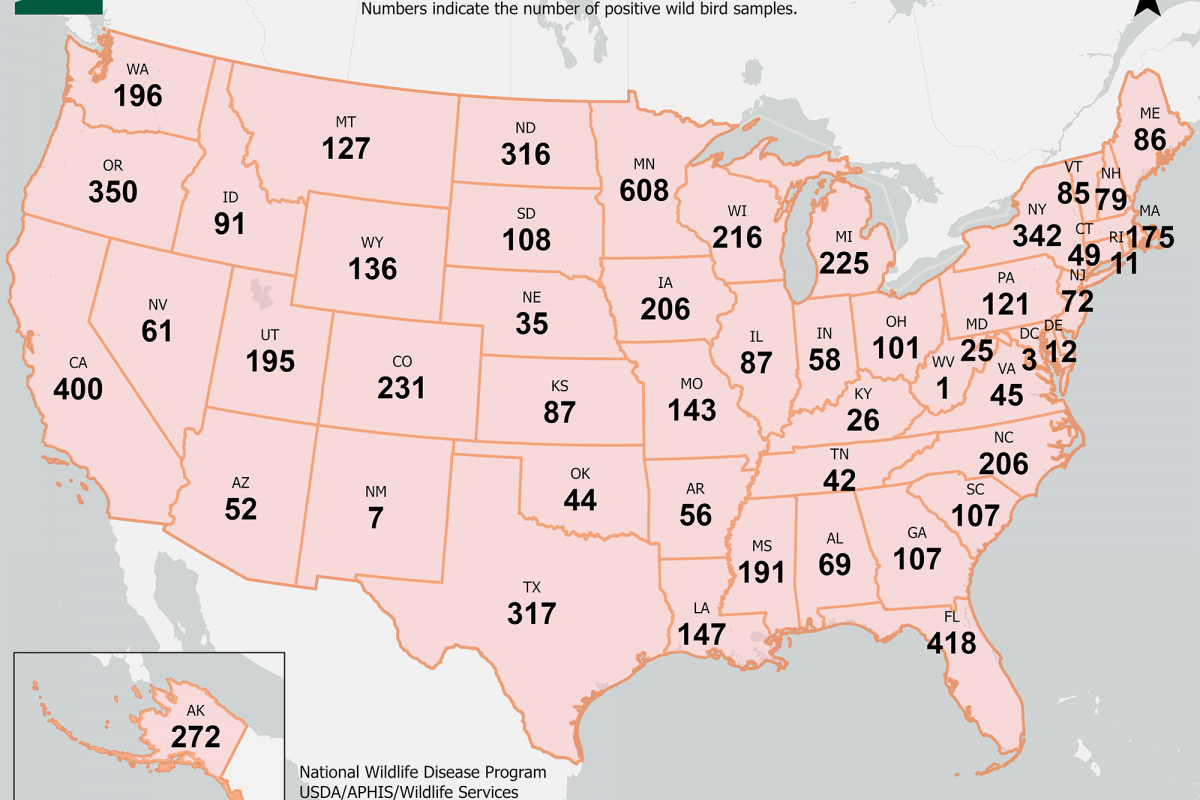
During this bird flu outbreak, there have been 7,105 virus detections in 47 U.S. states.
As of July 12, 2023, the U.S. government has stockpiled and approved avian influenza vaccines.